
Congenital heart disease in women becoming pregnant represents a challenge for cardiologist: a narrative review
Introduction
People born with a congenital heart defect (CHD) are by far living longer due to the steady progress in their surgical, interventional, and medical treatment. As such, when focusing on the whole population of subjects living with a CHD, nowadays those who are adult (ACHD, adult congenital heart disease) outnumber newborns and children affected by the same condition. In fact, the ratio between ACHD and paediatric patients is 70% vs. 30% currently, whereas in the past the latter represented the vast majority of the patients (1,2).
Such a change in epidemiology, with adult subjects with CHD presenting in increasing numbers at advanced ages, elderly included, is testified by the fact that the previously widely used acronym GUCH (grown-up congenital heart) is no longer considered appropriate and has been replaced by ACHD. The latter was used in the 2008 American Heart Association/American College of Cardiology (AHA/ACC) Guidelines on CHD for the first time (3). Then, for the sake of uniformity, the term ACHD appeared in the 2020 European Society of Cardiology (ESC) Guidelines as well (4).
Concerning women, being adult often implies developing a huge desire of maternity. Unfortunately, cardiac disease is still the first cause of death among pregnant women. Cardiovascular diseases complicate 1–4% of all pregnancies and, among these diseases, approx. 80% are congenital (5,6).
Pregnancy is characterized by multiple significant haemodynamic changes like increased circulating blood volume, raised stroke volume and maternal heart rate, reduced peripheral vascular resistances and so on (7). See Table 1. As such, becoming pregnant while ventricular systolic function is reduced, pulmonary pressure increased or with a circulation supported by a single ventricle can be really challenging. Not only that, but even foetal mortality is not negligible, with an average rate of miscarriage of 15%. The latter can reach a peak of 40% in ladies with a single ventricle physiology corrected according to Fontan procedure or in those who are cyanotic (8). Also the risk of CHD recurrence cannot be ignored, especially for pathologies involving the left and right ventricular outflow tracts as well as septal defects (7).
Table 1
| Stage | Haemodynamics |
|---|---|
| Early and intermediate pregnancy | Increased blood volume |
| Increased stroke volume | |
| Increased maternal heart rate | |
| Increased cardiac contractility (all over pregnancy. It triggers mild left ventricular hypertrophy) | |
| Decreased blood pressure | |
| Decreased vascular peripheral resistance | |
| Decreased haematocrit | |
| Decreased blood viscosity | |
| Delivery | Increased cardiac output mostly as a consequence of increased stroke volume |
| Increased blood pressure | |
| Postpartum | Increased plasmatic volume |
| Increased venous preload | |
| Increased cardiac output within the first hours following delivery. It comes back to baseline 2 weeks after delivery |
The list of the major challenges encountered in the management of ACHD women is summarised in Table 2. I present this article in accordance with the Narrative Review reporting checklist (available at https://jxym.amegroups.com/article/view/10.21037/jxym-22-34/rc)
Table 2
| Heart failure (with left ventricular ejection fraction less than 45%, systemic right ventricle, univentricular heart, severe aortic stenosis, severe aortic coarctation moderate or severe mitral valve stenosis, severe mitral valve regurgitation) |
| Arrhythmias (virtually in all congenital heart disease, most of all if surgically treated because of the presence of scar) |
| Thromboembolism (with left ventricular ejection fraction less than 25%, systemic right ventricle, univentricular heart, mechanical valve, pulmonary hypertension) |
| Aortic dissection (with Marfan, Ehler-Danlos, Loeys-Dietz and Turner syndromes, bicuspid aortic valve) |
Methods
A literature search was carried out on PubMed and Scopus using the Mesh terms “adult congenital heart disease”, “pregnancy”, “delivery”, “miscarriage”, “risk stratification”, “counselling” and their combination. The search strategy is summarised in Table 3. This review aims at discussing risk stratification, need for counselling regarding the maternal risk and the risk of the disease recurrence, multidisciplinary team involvement and management of pregnancies in some specific CHD.
Table 3
| Items | Specification |
|---|---|
| Date of search | August 2022 |
| Databases and other sources searched | PubMed and Scopus |
| Search terms used | “adult congenital heart disease”, “pregnancy”, “delivery”, “miscarriage”, “risk stratification”, “counselling” |
| Timeframe | From PubMed inception to August 2022 |
| Inclusion and exclusion criteria | Inclusion criteria: English language (at least the abstract). Exclusion criteria: vase reports |
| Selection process | The author (PPB) |
The Registry Of Pregnancy And Cardiac disease (ROPAC)
All the current information concerning ACHD women who are pregnant come out of the ROPAC. It was established in 2007 with the involvement of many centres, mostly European, and more than 6,000 pregnant women are enrolled so far. The vast majority of them are ACHD (57%), while 29% are affected by valvar disease (9). As per the data extracted from the ROPAC, maternal mortality is higher in the patients with pulmonary arterial hypertension (PAH) (+0.6% compared to the general population). Heart failure was observed in 11% of the sample and significant arrhythmias in 2%. The main risk factors for maternal complications during pregnancy are pre-pregnancy cardiac insufficiency with a functional class New York Heart Association (NYHA) >2, systemic ventricle ejection fraction <40%, risk class IV according to the modified World Health Organization classification (m-WHO), and anticoagulant therapy (9).
Risk stratification
Maternal and foetal risk of adverse events should be quantified before getting pregnant (10).
Many scoring systems have been proposed in the field. AHA/ACC (3), Canadian Cardiovascular Society (11), and ESC (4) Guidelines examine the cardiovascular risk during pregnancy for each CHD. The Guidelines are based on retrospective studies enrolling a limited number of patients. Again, the same CHD may present differently from a clinical point of view. Two scoring systems which take into account the maternal risk have been proposed: CARPREG risk score and ZAHARA score.
The CARPREG (Cardiac Disease in Pregnancy) is a Canadian multicentre prospective study evaluating risk and outcome of pregnant ladies, 75% of whom were ACHD (6). It proved to be very efficient in predicting maternal risk, but the latter may be overestimated (12,13). Furthermore, women with PAH and aortopathies are not included in the sample size. The higher the CARPREG score, the more increased the risk of post-delivery maternal adverse events as well (14).
The scoring system ZAHARA is based on a European study which enrolled just ACHD women, while the CARPREG involved the pregnant ladies with congenital as well as acquired diseases (12). There are no other studies aimed at validating the ZAHARA score and this is a significant limitation. PAH and aortic dilatation are underrepresented.
Based on these premises, the most useful scoring system is likely to be the modified WHO classification, which has been promoted since the release of the European Society of Cardiology (ESC) Guidelines for the management of cardiovascular diseases during pregnancy. All maternal risk factors have been taken together, including background and comorbidities (15,16). Four risk classes have been identified according to the increased risk:
- m-WHO I: very low risk pregnancies. One or two examinations amid pregnancy are enough;
- m-WHO II: pregnancy is at low-to-moderate risk. The suggested follow-up is made of an examination every trimester;
- m-WHO III: there is a significantly increased risk of maternal mortality or severe morbidity. The rate of adverse events is 19–27%. Pregnancy is advised against. If the patient wants to proceed with pregnancy at her own risk, she should be seen every month or 2 months;
- m-WHO IV: the risk of maternal mortality or severe morbidity is extremely high. The rate of adverse events is 40–100%. Pregnancy is contraindicated. If the patient wants to proceed with pregnancy at her own risk, she should be seen every month or 2 months.
All four m-WHO risk classes are very well correlated with the risk of maternal, foetal, and obstetric complications (15).
Pre-pregnancy exercise stress testing has been suggested to identify ACHD women at increased risk of cardiac complications. In fact, chronotropic incompetence during exercise proved to be an independent predictor of adverse events such as heart failure and arrhythmias in those ladies who failed to adequately increase heart rate on exertion (17).
Counselling and multidisciplinary team involvement
Counselling plays a pivotal role in ACHD women who are pregnant to help them and the couple in coping with such a stressful situation. It can be subdivided into genetic, cardiogical, and obstetric counselling which in turn implies the involvement of a multidisciplinary team (gynecologist, midwife, cardiologist, anaesthesiologist, neonatologist) (18). This is with the aim of letting the couple know about the maternal and foetal risk along with the risk of obstetric complications.
Genetic counselling is mainly focused on evaluating if maternal CHD is isolated or part of a syndromic spectrum. Clinical examination is aimed at identifying any signs (e.g., dysmorphism and/or non-cardiac congenital malformations) which may suggest the occurrence of a syndrome. In addition, genetic characterisation is crucial in estimating the risk of recurrence. Ferencz et al. found extracardiac malformations in 30% of their case series. In non-syndromic patients the risk of recurrence was 4.9% with a peak in those with hypoplastic left heart syndrome (13%) and aortic coarctation (8%) (19). Nora suggested that genetic inheritance is multifactorial and depends on the interaction between genetic and environment. The risk of recurrence varies and is 3% when parents are healthy and not consanguineous with just one child with CHD. It increases to 10% when also the second child has CHD (20). The risk is higher when the person with CHD is the mother rather than the father (21,22). It may at least in part explained by cytoplasmic heredity and is particularly significant in aortic stenosis and coarctation, pulmonary stenosis, septal defects, and atrioventricular defects (23). Specific single genes have been identified in 15–20% of CHD, like Tetralogy of Fallot, atrioventricular defects, interatrial septal defect, hypoplastic left heart syndrome, and left ventricular outflow tract obstruction. However, concerning the risk of recurrence, genetic penetrance is incomplete and expressivity is variable (24). If a specific monogenic syndrome is suspected, genomic sequencing, with or without multiplex ligation probe amplification, and array-CGH analysis are indicated (25). Genetic analysis is lengthy. This is the reason why genetic counselling should be done before getting pregnant. If the ACHD woman is already pregnant, chorionic villus sampling and amniocentesis are suggested (21).
Cardiologic counselling is complex and should be based on a personalized (“tailored”) approach with the aim of planning pregnancy (26). This is very important because of two reasons, that is suggesting becoming pregnant before a worsening in clinical conditions occurs or eventually fixing any residual complication before getting pregnant. Again, in ACHD women in risk class m-WHO IV pregnancy should be strongly discouraged (7).
Some Authors think that pre-conception counselling, especially for moderate-to-high risk ACHD, should start since adolescence, including providing information about contraception. ACHD women, even those with complex CHD, are often asymptomatic or with few symptoms and think that there are no contraindication concerning pregnancy (27). Generally speaking, valvar insufficiency, even when it is severe, is well tolerated amid pregnancy, since peripheral vascular resistance (afterload) diminishes. Low dose diuretic therapy can be provided to reduce volume overload as well (28). Conversely stenosis is poorly tolerated and may lead to pulmonary oedema (29). Cardiac contractility should be monitored since it is crucial in predicting the outcome of pregnancy (9).
Also obstetric counselling to evaluate foetal risk should be provided before planning pregnancy (10). The higher the m-WHO class, the higher the risk of obstetric complications (9). The averaged risk of miscarriage in ACHD is 15% with a peak of 40% in Fontan patients (8). Maternal worsening triggers foetal distress and in turn prematurity at birth, low birthweight, babies born small for gestational age, and need for caesarean section (9). Environmental (epigenetic) factors like advanced maternal age, smoking habit, multiparity pregnancy increase the above stated bad foetal outcomes (30,31). The frequency of controls during pregnancy depends on each patient’s m-WHO risk class. Owing to the risk of recurrence, foetal echocardiography should be done along with obstetric ultrasounds scan for colour Doppler flowmetry. Timing and modality of delivery should be planned no later than at the beginning of the third trimester (7).
Specific congenital heart diseases and pregnancy
The AHA/ACC Guidelines subdivide CHD into mild, moderate, and elevated complexity (3,32). Surgically treated Tetralogy of Fallot and univentricular heart after Fontan repair are the most frequently ACHD encountered in clinical practice (33,34).
In tetralogy of Fallot, post-surgical iatrogenic pulmonary valve incompetence leads to a progressive right ventricular dilatation (volume overload) and, in turn, to impaired contractility (35,36). Pregnancy is low-risk in women without any significant complications after surgery and with preserved right ventricular systolic function (37,38). In symptomatic ladies, pulmonary valve replacement should be done before pregnancy (4). Pulmonary valve regurgitation is correlated with intrauterine growth retardation and low weight at birth (39). Pulmonary valve stenosis is poorly tolerated since increased blood volume causes an increase in preload and a surge in the gradient across the valve (39). Cardiac complications during pregnancy are seen in 7–10% and include supraventricular arrhythmias, symptomatic right atrial failure, and pulmonary embolism (40). Obstetric issues complicate up to 30% of pregnancies (39). In case of right ventricular dysfunction, caesarean section should be preferred to vaginal delivery (39).
In Fontan patients, single ventricle systolic function deteriorates with time (41). This occurs even faster with pregnancy-related volume overload. It makes pre-pregnancy asymptomatic patients becoming symptomatic (37,38). All pregnancies are at significantly increased-to-extremely high risk of maternal mortality and severe morbidity (m-WHO III and IV) because of the worsening in NYHA class and possible onset of arrhythmias The latter should be promptly treated with DC shock (4,42). Fontan women are often infertile, though delivery is not impossible (43). The risk of miscarriages is high (8). There is no universally accepted specific risk-scoring system for Fontan ladies who are pregnant. That used in the British Islands subdivides patients in low, intermediate, and very high risk on the basis of NYHA class, saturation on room air, VO2 max at cardiopulmonary exercise stress test, single ventricle systolic function, degree of atrioventricular valve incompetence, Fontan related complications, and signs of failure (44). As per the ESC Guidelines, pregnancy is contraindicated in Fontan patients with saturation lower than 85%, reduced ventricular function, moderate-to-severe atrioventricular valve incompetence, refractory arrhythmias, protein-losing enteropathy (4).
Systemic right ventricle is a condition which can be seen in natural history (congenitally corrected transposition of the great vessels, cc-TGV) or as a consequence of palliative surgery [(d-TGV), corrected according to Mustard or Senning procedure (atrial switch)] (45). As to cc-TGV, the main complications during pregnancy are systemic right ventricular dysfunction, systemic tricuspid valve regurgitation, atrioventricular blocks (complete heart block included). Generally speaking, arrhythmias are treated with beta blockers, though complete heart block represents a possible adverse side effect (46,47). Regarding d-TGV after atrial switch operation, the main issues amid pregnancy are systemic right ventricular dysfunction, systemic tricuspid valve regurgitation, sinus node dysfunction (mostly in patients who are treated with beta blockers for arrhythmic troubles), baffle stenosis and leaks (48-50). All patients with a systemic right ventricle, in NYHA class III or IV, with systemic right ejection fraction less than 40%, or severe tricuspid regurgitation should be advised against pregnancy (4).
CHD with left-to-right shunt like patent ductus arteriosus, interatrial septal defect, interventricular septal defect are usually well tolerated throughout pregnancy, unless pulmonary arterial pressure is increased or the left ventricle is dilated thus triggering arrhythmias (51-53). If irreversible PAH affects the patients (Eisenmenger syndrome) the risk of maternal death is 30% and pregnancy is strongly contraindicated (4,54). Even the risk of poor foetal outcome in terms of intrauterine growth retardation, prematurity at birth, and death is very high (55). Should the couple decide to proceed with pregnancy, delivery is always through caesarean section (week 32–34). The risk of maternal mortality persists even after delivery. As such, women should stay admitted to hospital 2 weeks following carriage (56,57).
Also aortic artery size over 40 mm in Marfan syndrome or other heritable thoracic aortic diseases is a risk factor for aortic dissection (58).
Foetal risk
Prenatal care
As above stated, with pregnant ladies affected by CHD there is a risk of disease recurrence, intrauterine growth restriction/preterm birth, and miscarriage (59). The risk is higher compared to healthy peers and is consistent with the severity of the maternal CHD as well as their m-WHO class (59). The risk of miscarriage in ACHD pregnant ladies is significantly increased compared with the 10% which is expected in the general population. Prematurity at birth can often occur as a consequence of spontaneous early labour or the need for bringing delivery forward due to a worsening in maternal conditions. Not only the increased incidence of neonatal complications is linked with the severity of maternal disease (NYHA functional class >2, cyanosis, outflow tract obstruction, mechanical valves), but also with comorbidities like maternal age, smoking habit, multiple pregnancy, anticoagulant therapy (30,31).
The known relationship between maternal CHD complexity and adverse foetal/neonatal outcomes highlights that a multidisciplinary approach is crucial in keeping maternal haemodynamic stable as much as possible during pregnancy. In fact, foetal/neonatal outcomes depend on utero-placental perfusion (7).
Assessment of foetal vitality
Owing to the increased risk of CHD recurrence and miscarriage, undergoing an obstetric ultrasound scan as well as a foetal echocardiogram at week 19–21 of gestation is mandatory. Again, an additional scan at week 28–30 is due in all ladies taking beta-blocker therapy to check foetal biometry and maternal-foetal Doppler velocimetry which may be negatively influenced by the therapy. Timing and delivery modality can be planned since the beginning of the third trimester of pregnancy (7).
Management of delivery
Labour is characterised by haemodynamic changes to provide the foetus with an adequate blood flow. As such, blood flow toward the uterus increases 10-fold compared to pre-pregnancy and that to the mother’s kidneys increases of 30% (60). ACHD women display a reduced ability to adapt to these changes thereby increasing the risk of the onset of heart failure, arrhythmias, and death. Again, uterine contractions and Valsalva maneuver during delivery make the above stated changes even more strong with a 180% increase in cardiac output compared to pre-pregnancy and a rise in blood pressure (60). The timing of delivery depends on the balance between maternal and preterm birth risk. This is the reason why a multidisciplinary approach is needed (10,61). With the aim of minimizing the risk of heart failure, delivery over 40 weeks of gestation is not recommended. The gold standard for delivery is week 37–38, but for those ladies with complex and hypercomplex CHD delivery can be brought forward. In these cases, prophylactic corticosteroids are needed to accelerate foetal lung maturity and prevent respiratory distress syndrome (10,31,61,62). Vaginal delivery is preferred. Caesarian section is limited to few indications like in patients who are still taking anticoagulants, in Marfan patients with aortic dilatation/dissection, severe aortic stenosis, Eisenmenger syndrome (62). Epidural analgesia is preferred. ACHD women should be monitored closely even after delivery (61,62).
The foetal echocardiographic parameters which are assessed in pregnant women with CHD are reported in Table 4.
Table 4
| 1. Cardiac situs |
| 2. Four-chamber view of the heart |
| 3. Aortic valve and left ventricular outflow tract |
| 4. Pulmonary valve and right ventricular outflow tract |
| 5. The three-vessel and three-vessel tracheal views |
| 6. Pulmonary venous return |
| 7. Transverse aortic arch |
| 8. Transverse ductal arch |
| 9. Aortic isthmus in three-vessel view and longitudinally |
| 10. Bicaval view |
| 11. Longitudinal view of the aortic arch, head, and neck vessels |
| 12. Longitudinal view of the ductal arch |
| 13. Short-axis of the atrioventricular valves |
| 14. Heart rate and rhythm |
| 15. Aortic and pulmonary valve pulsed-wave Doppler |
| 16. Mitral and tricuspid valve pulsed-wave Doppler |
| 17. Pulmonary venous pulsed-wave Doppler |
| 18. Cardiac contractility |
Conclusions
Pregnancy can be afforded by almost ACHD women, though it is not without any risk. Always it is contraindicated in patients with PAH, irrespective of its aetiology. Also patients with single ventricle and Fontan physiology can become pregnant and deliver successfully.
Pre-pregnancy risk should be discussed in specialised centres, especially for patients in risk class m-WHO III and IV. The approach should be personalised. Follow-up during pregnancy and delivery should be provided in any case (see Figure 1).
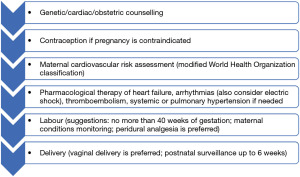
In spite of a number of scientific papers stating the need for multidisciplinary approach for women with CHD, there is a lack of evidence concerning structure or pathways of multidisciplinary working, nor impact on maternal or newborn outcomes. Discussion should at least involve the evaluation of maternal and foetal risks, recurrence risk, evaluation of medications, options for contraception and risks of assisted reproductive therapy. These are the key principles for the management of pregnant women with CHD along with pre-conception genetic/cardiac/obstetric counselling, tight follow-up according to the m-WHO class of risk, therapy of complications during pregnancy, and vaginal delivery when possible. Apart from rare exceptions, vaginal delivery should be preferred, as in the trade-off with caesarean section, complications (haemorrhage, infection, hemodynamic disturbances, coagulation disorders) are less frequent (4).
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The author has completed the Narrative Review reporting checklist. Available at https://jxym.amegroups.com/article/view/10.21037/jxym-22-34/rc
Conflicts of Interest: The author has completed the ICMJE uniform disclosure form (available at https://jxym.amegroups.com/article/view/10.21037/jxym-22-34/coif). PPB serves as an unpaid editorial board member of Journal of Xiangya Medicine from January 2022 to December 2023.
Ethical Statement: The author is accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bassareo PP, Mcmahon CJ, Prendiville T, et al. Planning Transition of Care for Adolescents Affected by Congenital Heart Disease: The Irish National Pathway. Pediatr Cardiol 2023;44:24-33. [Crossref] [PubMed]
- Taylor J. Congenital heart disease is no longer a paediatric specialty. Eur Heart J 2014;35:673-4. [PubMed]
- Stout KK, Daniels CJ, Aboulhosn JA, et al. 2018 AHA/ACC Guideline for the Management of Adults With Congenital Heart Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2019;139:e698-800. [PubMed]
- Baumgartner H, De Backer J, Babu-Narayan SV, et al. 2020 ESC Guidelines for the management of adult congenital heart disease. Eur Heart J 2021;42:563-645. [Crossref] [PubMed]
- Curtis SL, Marsden-Williams J, Sullivan C, et al. Current trends in the management of heart disease in pregnancy. Int J Cardiol 2009;133:62-9. [Crossref] [PubMed]
- Siu SC, Sermer M, Colman JM, et al. Prospective multicenter study of pregnancy outcomes in women with heart disease. Circulation 2001;104:515-21. [Crossref] [PubMed]
- Bianca I, Geraci G, Gulizia MM, et al. ANMCO/SICP/SIGO Consensus document: Pregnancy and congenital heart disease. G Ital Cardiol (Rome) 2016;17:687-755. [PubMed]
- Drenthen W, Pieper PG, Roos-Hesselink JW, et al. Outcome of pregnancy in women with congenital heart disease: a literature review. J Am Coll Cardiol 2007;49:2303-11. [Crossref] [PubMed]
- Roos-Hesselink J, Baris L, Johnson M, et al. Pregnancy outcomes in women with cardiovascular disease: evolving trends over 10 years in the ESC Registry Of Pregnancy And Cardiac disease (ROPAC). Eur Heart J 2019;40:3848-55. [Crossref] [PubMed]
- Pieper PG. Pre-pregnancy risk assessment and counselling of the cardiac patient. Neth Heart J 2011;19:477-81. [Crossref] [PubMed]
- Howlett JG, McKelvie RS, Costigan J, et al. The 2010 Canadian Cardiovascular Society guidelines for the diagnosis and management of heart failure update: Heart failure in ethnic minority populations, heart failure and pregnancy, disease management, and quality improvement/assurance programs. Can J Cardiol 2010;26:185-202. [Crossref] [PubMed]
- Drenthen W, Boersma E, Balci A, et al. Predictors of pregnancy complications in women with congenital heart disease. Eur Heart J 2010;31:2124-32. [Crossref] [PubMed]
- Jastrow N, Meyer P, Khairy P, et al. Prediction of complications in pregnant women with cardiac diseases referred to a tertiary center. Int J Cardiol 2011;151:209-13. [Crossref] [PubMed]
- Balint OH, Siu SC, Mason J, et al. Cardiac outcomes after pregnancy in women with congenital heart disease. Heart 2010;96:1656-61. [Crossref] [PubMed]
- Regitz-Zagrosek V, Roos-Hesselink JW, Bauersachs J, et al. 2018 ESC Guidelines for the management of cardiovascular diseases during pregnancy. Eur Heart J 2018;39:3165-241. [Crossref] [PubMed]
- Thorne S, MacGregor A, Nelson-Piercy C. Risks of contraception and pregnancy in heart disease. Heart 2006;92:1520-5. [Crossref] [PubMed]
- Lui GK, Silversides CK, Khairy P, et al. Heart rate response during exercise and pregnancy outcome in women with congenital heart disease. Circulation 2011;123:242-8. [Crossref] [PubMed]
- Greutmann M, Pieper PG. Pregnancy in women with congenital heart disease. Eur Heart J 2015;36:2491-9. [Crossref] [PubMed]
- Ferencz C, Boughman JA, Neill CA, et al. Congenital cardiovascular malformations: questions on inheritance. Baltimore-Washington Infant Study Group. J Am Coll Cardiol 1989;14:756-63. [Crossref] [PubMed]
- Nora JJ. Multifactorial inheritance hypothesis for the etiology of congenital heart diseases. The genetic-environmental interaction. Circulation 1968;38:604-17. [Crossref] [PubMed]
- Burn J, Brennan P, Little J, et al. Recurrence risks in offspring of adults with major heart defects: results from first cohort of British collaborative study. Lancet 1998;351:311-6. [Crossref] [PubMed]
- Fesslova V, Brankovic J, Lalatta F, et al. Recurrence of congenital heart disease in cases with familial risk screened prenatally by echocardiography. J Pregnancy 2011;2011:368067. [Crossref] [PubMed]
- Nora JJ, Nora AH. Maternal transmission of congenital heart diseases: new recurrence risk figures and the questions of cytoplasmic inheritance and vulnerability to teratogens. Am J Cardiol 1987;59:459-63. [Crossref] [PubMed]
- Digilio MC, Silvestri LM, Dallapiccola B, et al. Le basi genetiche delle cardiopatie congenite. Cardiologia Pediatrica 2014;175:173-86.
- Erdogan F, Larsen LA, Zhang L, et al. High frequency of submicroscopic genomic aberrations detected by tiling path array comparative genome hybridisation in patients with isolated congenital heart disease. J Med Genet 2008;45:704-9. [Crossref] [PubMed]
- Hinton RB Jr, Martin LJ, Tabangin ME, et al. Hypoplastic left heart syndrome is heritable. J Am Coll Cardiol 2007;50:1590-5. [Crossref] [PubMed]
- Montis S, Bassareo PP, Follese C, et al. Counseling and informed consent: the experience in a pediatric cardiology unit. How to communicate a pathological diagnosis. Pediatr Med Chir 2010;32:206-10. [PubMed]
- Otto CM. Clinical practice. Evaluation and management of chronic mitral regurgitation. N Engl J Med 2001;345:740-6. [Crossref] [PubMed]
- Arnolds DE, Dean C, Minhaj M, et al. Cardiac Disease in Pregnancy: Hypertrophic Obstructive Cardiomyopathy and Pulmonic Stenosis. J Cardiothorac Vasc Anesth 2021;35:3806-18. [Crossref] [PubMed]
- Gandhi M, Martin SR. Cardiac disease in pregnancy. Obstet Gynecol Clin North Am 2015;42:315-33. [Crossref] [PubMed]
- Lindley KJ, Conner SN, Cahill AG. Adult Congenital Heart Disease in Pregnancy. Obstet Gynecol Surv 2015;70:397-407. [Crossref] [PubMed]
- D'Alto M, Budts W, Diller GP, et al. Does gender affect the prognosis and risk of complications in patients with congenital heart disease in the modern era? Int J Cardiol 2019;290:156-61. [Crossref] [PubMed]
- Morris CD, Menashe VD. Recurrence of congenital heart disease in offspring of parents with surgical correction Clin Res 1985;33:68A. [abstract].
- Bhatt AB, DeFaria Yeh D. Pregnancy and Adult Congenital Heart Disease. Cardiol Clin 2015;33:611-23. ix. [Crossref] [PubMed]
- Bassareo PP, Deidda M, Calcaterra G, et al. Right ventricular diastolic function in post-surgical Tetralogy of Fallot patients: A pilot study to make a comparison between echocardiography and cardiac MRI. Int J Cardiol Congenit Heart Dis 2021;4:100135. [Crossref]
- Bassareo PP, Saba L, Marras AR, et al. Altered Aortic Upper Wall TDI Velocity Is Inversely Related with Left Ventricular Diastolic Function in Operated Tetralogy of Fallot. Congenit Heart Dis 2016;11:598-605. [Crossref] [PubMed]
- de Souza JA, Martinez EE Jr, Ambrose JA, et al. Percutaneous balloon mitral valvuloplasty in comparison with open mitral valve commissurotomy for mitral stenosis during pregnancy. J Am Coll Cardiol 2001;37:900-3. [Crossref] [PubMed]
- Hameed A, Yuodim K, Mahboob A, et al. Effect of the severity of pulmonary stenosis on pregnancy outcomes: a case-control study Am J Obstet Gynecol 2004;191:S93. [abstract]. [Crossref]
- Gelson E, Gatzoulis M, Steer PJ, et al. Tetralogy of Fallot: maternal and neonatal outcomes. BJOG 2008;115:398-402. [Crossref] [PubMed]
- Veldtman GR, Connolly HM, Grogan M, et al. Outcomes of pregnancy in women with tetralogy of Fallot. J Am Coll Cardiol 2004;44:174-80. [Crossref] [PubMed]
- Bassareo PP, Tumbarello R, Piras A, et al. Evaluation of regional myocardial function by Doppler tissue imaging in univentricular heart after successful Fontan repair. Echocardiography 2010;27:702-8. [Crossref] [PubMed]
- Moore JP, Mondésert B, Lloyd MS, et al. Clinical Experience With the Subcutaneous Implantable Cardioverter-Defibrillator in Adults With Congenital Heart Disease. Circ Arrhythm Electrophysiol 2016;9:e004338. [Crossref] [PubMed]
- Wichert-Schmitt B, D'Souza R, Silversides CK. Reproductive Issues in Patients With the Fontan Operation. Can J Cardiol 2022;38:921-9. [Crossref] [PubMed]
- Arif S, Chaudhary A, Clift PF, et al. Pregnancy outcomes in patients with a fontan circulation and proposal for a risk-scoring system: single centre experience. Journal of Congenital Cardiology 2017;1:10. [Crossref]
- Bassareo PP, Saba L, Solla P, et al. Factors influencing adaptation and performance at physical exercise in complex congenital heart diseases after surgical repair. Biomed Res Int 2014;2014:862372. [Crossref] [PubMed]
- Kowalik E, Klisiewicz A, Biernacka EK, et al. Pregnancy and long-term cardiovascular outcomes in women with congenitally corrected transposition of the great arteries. Int J Gynaecol Obstet 2014;125:154-7. [Crossref] [PubMed]
- Therrien J, Barnes I, Somerville J. Outcome of pregnancy in patients with congenitally corrected transposition of the great arteries. Am J Cardiol 1999;84:820-4. [Crossref] [PubMed]
- Cataldo S, Doohan M, Rice K, et al. Pregnancy following Mustard or Senning correction of transposition of the great arteries: a retrospective study. BJOG 2016;123:807-13. [Crossref] [PubMed]
- Trigas V, Nagdyman N, Pildner von Steinburg S, et al. Pregnancy-related obstetric and cardiologic problems in women after atrial switch operation for transposition of the great arteries. Circ J 2014;78:443-9. [Crossref] [PubMed]
- Canobbio MM, Morris CD, Graham TP, et al. Pregnancy outcomes after atrial repair for transposition of the great arteries. Am J Cardiol 2006;98:668-72. [Crossref] [PubMed]
- Rashkind WJ, Mullins CE, Hellenbrand WE, et al. Nonsurgical closure of patent ductus arteriosus: clinical application of the Rashkind PDA Occluder System. Circulation 1987;75:583-92. [Crossref] [PubMed]
- Metcalfe J, McAnulty JH, Ueland K. Cardiac disease and pregnancy: physiology and management. 2nd edition. Boston, MA: Little, Brown, 1986.
- Presbitero P, Somerville J, Stone S, et al. Pregnancy in cyanotic congenital heart disease. Outcome of mother and fetus. Circulation 1994;89:2673-6. [Crossref] [PubMed]
- Galiè N, Humbert M, Vachiery JL, et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Heart J 2016;37:67-119. [Crossref] [PubMed]
- Bédard E, Dimopoulos K, Gatzoulis MA. Has there been any progress made on pregnancy outcomes among women with pulmonary arterial hypertension? Eur Heart J 2009;30:256-65. [Crossref] [PubMed]
- Kiely DG, Condliffe R, Webster V, et al. Improved survival in pregnancy and pulmonary hypertension using a multiprofessional approach. BJOG 2010;117:565-74. [Crossref] [PubMed]
- Jaïs X, Olsson KM, Barbera JA, et al. Pregnancy outcomes in pulmonary arterial hypertension in the modern management era. Eur Respir J 2012;40:881-5. [Crossref] [PubMed]
- Pyeritz RE. Maternal and fetal complications of pregnancy in Marfan syndrome. Am J Med 1981;71:784-90. [Crossref] [PubMed]
- Roos-Hesselink JW, Ruys TP, Stein JI, et al. Outcome of pregnancy in patients with structural or ischaemic heart disease: results of a registry of the European Society of Cardiology. Eur Heart J 2013;34:657-65. [Crossref] [PubMed]
- Hunter S, Robson SC. Adaptation of the maternal heart in pregnancy. Br Heart J 1992;68:540-3. [Crossref] [PubMed]
- European Society of Gynecology (ESG). ESC Guidelines on the management of cardiovascular diseases during pregnancy: the Task Force on the Management of Cardiovascular Diseases during Pregnancy of the European Society of Cardiology (ESC). Eur Heart J 2011;32:3147-97. [Crossref] [PubMed]
- Linee guida SIEOG. Cento (Ferrara): Editeam Publisher; 2015.
Cite this article as: Bassareo PP. Congenital heart disease in women becoming pregnant represents a challenge for cardiologist: a narrative review. J Xiangya Med 2023;8:10.


