
Blood purification for perioperative management of cardiac surgery
Introduction
Cardiopulmonary bypass (CPB) in cardiac surgery is closely associated with several adverse effects, including hemodilution, coagulopathy and activation of a systemic inflammatory response due to operative trauma, ischemia-reperfusion injury, endotoxemia and blood contact with synthetic surfaces of the circuit. This inflammatory response increased vascular permeability and interstitial oedema, leading to cardiac, respiratory, renal, hepatic, and even multiorgan dysfunction (1). One of the measures to counteract these CPB-related pathologic syndrome is perioperative blood purification, which is mandatory in patients with advanced renal failure.
Blood purification or renal replacement therapy (RRT) is a technique of treatment with extracorporeal circulation to correct body fluids, to remove etiological substances in blood, and to replenish deficient substances. It is currently utilized to manage acute kidney injury (AKI) and other organ dysfunction syndromes in critically ill patients, including acute heart failure, acute pancreatitis, fulminant hepatitis, acute drug poisoning, and sepsis (2). It also plays an important role in perioperative management of highly invasive cardiac surgery, especially in the current era of an increasing number of older patients with multiple comorbidities are undergoing cardiac surgery.
This review provides an up-to-date summary of four roles of blood purification or RRT in perioperative management of cardiac surgery as follows: (I) simplified intermittent hemodialysis (HD) in the patients with chronic kidney disease (CKD) dependent on HD; (II) continuous or intermittent RTT for postoperative AKI; (III) aggressive dilution ultrafiltration (DUF) during CPB; and (IV) continuous RTT after surgery for the patients with fluid overload and pulmonary edema, even if urine output is secured.
Management of HD-dependent patients with CKD
Backgrounds
The number of adult patients with CKD in Japan is estimated to be approximately 13.3 million (12.9% of the adult population) (3), and the number continues to increase, associated with aging population and increased patients with diabetic nephropathy. The number of chronic HD patients in Japan also continues to increase every year; as of the end of 2018, it had reached 339,841 patients, representing 2,688 patients per million population (4). The mean age was 68.75 years, and diabetic nephropathy was the most common primary disease among the prevalent dialysis patients (39.0%), followed by chronic glomerulonephritis (26.8%) and nephrosclerosis (10.8%). The causes of death in HD patients were heart failure (23.5%), infectious disease (21.3%), malignancy (8.4%), and cerebrovascular disease (6.0%), respectively. Therefore, more HD patients with increased age are more frequently the candidates for cardiac surgery.
Simplified intermittent HD
The patients on chronic HD have a variety of comorbidities, including diabetes, peripheral artery disease, cerebral infarction, anemia, bleeding tendency, infectious disease, malnutrition, and chronic hepatitis (5). Blood purification provides appropriate perioperative management of fluid balance, electrolytes, uremia, and metabolic acidosis in such high-risk HD patients. Although intraoperative HD (6) and postoperative continuous venovenous hemodiafiltration (CVVH) (7) have been reported, the simplified intermittent HD (8) is uncomplicated and useful as follows:
- Preoperative HD on the consecutive 2 days before surgery is performed using a low-potassium dialysate containing 1.5 mEq/L of potassium, in contrast to a potassium concentration of 2.0 mEq/L in the standard dialysate, to obtain a serum potassium of 3.0 mEq/L.
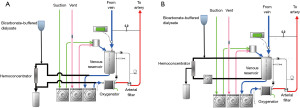
- During CPB, hemofiltration is performed, as well as in non-HD patients, using a hemoconcentrator incorporated into the CPB circuit. Blood from the venous reservoir is passed through the hemoconcentrator, and then the hemoconcentrated blood enters the reservoir again or the oxygenator (Figure 1). A standard dialysis fluid is used for fluid replacement. The goal for discontinuation of CPB was to obtain a hemoglobin greater than 10.0 g/dL, a hematocrit greater than 30%, and serum potassium value less than 4.0 mEq/L by increased hemofiltration and blood transfusion.
- In patients undergoing off-pump surgery, any hemofiltration is not applied during the surgery.
- To avoid hyperkalemia due to potassium-rich cardioplegia, high volume blood transfusion, and atherosclerosis-related peripheral hypoperfusion, the red cell products are washed using an autologous cell salvage system (cell saver: Hemonetec, etc.) or potassium adsorption filter (Kawasumi Laboratories, Inc., Tokyo, Japan) is applied as needed.
- On the day of surgery, blood purification is not performed. The first postoperative HD was carried out on the next day of surgery in the intensive care unit (ICU) using a standard HD device via the arteriovenous fistula or shunt of the patient. We did not insert any additional catheters as a vascular access for intermittent HD. A low-flux polysulphone HD membrane with an active surface area of 1.3 m2 is usually used. Anticoagulation was achieved using a dose of 25 mg/h of nafamostat mesylate, followed by patient-adjusted regimen based on the activated clotting time (150 to 180 seconds). Blood flow was 200 mL/min and ultrafiltration (UF) rate are 250 to 500 mL/h to achieve a negative fluid balance for 4 hours. A bicarbonate-buffered dialysate (Kindary AF; Fuso Pharmaceutical, Ltd., Osaka, Japan) was used during the HD. Approximately 4 hours of HD was performed to obtain the potassium value less than 4.5 mEq/L. The amount of water removal is set with reference to intraoperative fluid balance, postoperative infusion volume, and chest X-ray findings.
- The second postoperative HD is usually carried out on the third postoperative day by the usual standard methods using heparin, followed intermittent regular HD every other day. When additional volume removal is desired, only UF is performed using a shunt. It is important to avoid rapid negative balance of fluid volume because there is a fetal risk of intestinal ischemia or non-occlusive mesenteric ischemia (NOMI) in dehydrated HD patients (9). Therefore, the patient’s weight is gradually decreased to reach the preoperative dry weight for 10 days after surgery.
- Only under unstable postoperative hemodynamic condition, a 12 Fr double-lumen catheter is newly inserted and CVVH is conducted using a high-flux polyacrylonitrile hemofilter with a surface area of approximately 0.6 m2. As used for HD in the ICU, nafamostat mesylate was used for anticoagulation. Hemodiafiltration was initially accomplished by using a blood flow rate of 100 mL/min, a bicarbonate-buffered dialysate flow rate of 500 mL/h, and an UF rate of 500 mL/h with no net fluid removal. After the hemodynamics became stable, the filtration rate and dialysate flow were adjusted to remove the fluid excess, followed by weaning from CVVH and institution of intermittent regular HD via the arteriovenous fistula or shunt.
Management of patients with postoperative AKI
Backgrounds
The incidence of AKI after cardiac surgery has been reported to be as high as 14%, depending on the definition of increases in serum creatinine and decreases in urine output over time (10-17), as shown in Table 1. Cardiac surgery-associated AKI is a serious complication and one of the stronger risk factors for in-hospital mortality and poor long-term prognosis in patients undergoing cardiac surgery (15,18). Currently, AKI is defined by the Kidney Disease Improving Global Outcomes (KDIGO) consensus (19).
Table 1
| First author | Country | Year | Patients | AKI definition | Incidence (%) | Risk factors |
|---|---|---|---|---|---|---|
| Parolari A (12) | Italy | 2012 | 3,219 | RIFLE | 8.9 | Age, diabetes, smoking, preoperative high Cr level, blood transfusion, aortic cross-clamp time, resumption of CPB, decreased urine output during CPB, use of cardiotonic/antiarrhythmic drugs after surgery |
| Rahmanian PB (13) | Germany | 2013 | 5,318 | Need for RRT | 5.5 | – |
| Lopez-Delgado JC (14) | Spain | 2013 | 2,940 | RIFLE | 14.0 | Prolonged CPB, use of vasoconstrictives, high lactate level |
| Dardashti A (15) | Sweden | 2014 | 5,746 | RIFLE | 10.8 | – |
| Ng SY (16) | Australia | 2014 | 28,422 | Cr >2.26 mg/dL or >2 times of preoperative Cr |
5.8 | Preoperative high Cr level, age, obesity, diabetes, infective endocarditis, shock, CPB time >180 min, blood transfusion |
| Sato Y (17) | Japan | 2015 | 1,688 | Need for RRT | 7.6 | Preoperative high Cr level, CPB, lower body surface area, lower left ventricular ejection fraction, lower albumin |
AKI, acute kidney injury; RIFLE, Risk, Injury, Failure, Loss of kidney function and End stage of kidney disease (11); Cr, serum creatinine; CPB, cardiopulmonary bypass; RRT, renal replacement therapy.
Risk factors for postoperative AKI include age, diabetes, obesity, low albumin, smoking, chronic obstructive pulmonary disease, poor preoperative renal function, low cardiac function, preoperative shock), and infectious endocarditis, vasoconstrictive drug use, blood transfusion, excessive anemia, blood dilution, longer cardiac ischemic time, and CPB time >160 min (12-18). Although diuretic use may be a risk factor for postoperative AKI, Gandhi et al. (20) analyzed 47 studies and reported that while there is little evidence that sustained diuretic administration avoids blood purification or RRT, timely administration does not lead to deterioration of renal function.
Indication for blood purification
In the presence of severe AKI with oliguria, hyperkalemia, acidosis, or uremia, RRT is mandatory. The practical indication for RRT is shown in Table 2. Although the frequency, modality, continuous or intermittent, and dose are controversial, continuous RRT or CVVH, providing more hemodynamic stability, is often used postoperatively (21). Trials of early vs. late timing of initiating RRT in AKI suggest a benefit to early initiation (22-24). However, as shown that an accelerated RRT was not associated with a lower risk of death at 90 days than a standard strategy among critically ill 3,000 patients with AKI (25), CVVH has never been consistently shown to improve outcomes of cardiac surgery patients.
Table 2
| (I) Hyperkalemia (>6.0 mEq/L) |
| (II) Advanced metabolic acidosis (arterial pH <7.20) |
| (III) Lower urine output <240 mL/12 h (even with furosemide of 80 mg) |
| (IV) Progressive increase in BUN and Cr |
| (V) Profound fluid overload resulting in pulmonary congestion CVP >15 mmHg PaO2/FiO2 <300 |
RTT, renal replacement therapy; CVVH, continuous venovenous hemodiafiltration; AKI, acute kidney injury; BUN, blood urea nitrogen; Cr, serum creatinine; CVP, central venous pressure; PaO2, arterial oxygen pressure; FiO2, fraction of inspired oxygen.
In cardiac surgery-associated AKI, the complications of RRT should be recognized, including catheter-related infection and bleeding due to anticoagulation and thrombocytopenia. However, we should not hesitate to introduce early RRT to correct excess fluid overload after invasive surgery.
Intermittent or continuous RRT?
There is a debate about the modes of RRT, intermittent or continuous. The former is mainly HD, while the latter has CVVH. A prospective randomized study (26) showed no significant difference between intermittent and continuous RRTs in renal prognosis and survival. The AKI Treatment Guidelines for KDIGO (19) advocates continuous RTT in the patients with unstable hemodynamics, increased intracranial pressure, and cerebral edema.
There are also several controversies about the modes of continuous RTT or CVVH, continuous hemofiltration (CHF), continuous HD (CHD), or continuous hemodiafiltration (CHDF) (27). Small molecular weight substances, including blood urea nitrogen (BUN), creatinine, uric acid, and potassium, are efficiently removed by diffusion rather than UF. Therefore, CHD is desirable for the removal of such small molecular weight substances. However, in continuous RRT with lower flow rate of the dialysate, there is mostly no difference among the three modes, CHF, CHD, or CHDF, in removal of small molecular weight substances. When compared in the conditions of the same blood purification volume, CHF, which filters many substances from small to medium molecular weight, is superior from the viewpoint of removal efficiency. However, longer CHF may result in clogging of membrane pores due to proteins adhesion. Therefore, CHF enabling sufficient blood flow is used from the viewpoint of removal efficiency, while CHDF with diffusion principle to avoid clogging in the circuit is used for longer durations.
As a new mode, sustained low-efficiency dialysis (SLED), a hybrid of CVVH and intermittent HD, via a double-lumen catheter is noticeable (28,29). An 8-hour SLED provides the solute removal with rapid normalization of uremia under hemodynamic stabilization, equivalent to CVVH, routinely without anticoagulation. SLED is delivered using the conventional HD machines and a dialyzer made of polysulfone. Blood flow is 200 mL/min and the dialysate flow is 300 mL/min. The UF rate set in SLED is less than 500 mL/h to achieve to the desirable dry weight.
UF doses in RRT
Since Ronco et al. (30) reported that at least 35 mL/kg/h is desirable, the optimal amount of blood purification in continuous RRT for AKI has been discussed. Some large-scale randomized controlled studies in ICU, including the Acute Renal Failure Trial Network (ATN) study (31) and Randomized Evaluation of Normal versus Augmented Level Renal Replacement Therapy (RENAL) study (32), showed that high doses (35 mL/kg/h) RRT does not improve prognosis compared to low-dose (25 mL/kg/h) RRT. The KIGO Guideline (19) recommend relatively low-dose UF, 20 to 25 mL/kg/h.
For cardiac surgery-associated AKI, Bent et al. (33) reported that early, high-volume CHF reduced the mortality rate to 40% in 65 postoperative AKI patients (2.1% of all patients) with predicted mortality rate of 66%. They initiated CHF with a blood flow rate of 200 to 250 mL/min and a UF volume of 2,000 mL/h (equivalent to 35 mL/kg/h) on an average of 2.38 days after surgery and continued CHF for 3.98 days. Li et al. (34) also treated 142 patients with AKI after cardiac surgery using CHF at low doses (1,000–1,200 mL/h) in 45 patients or high dose (3,000 mL/h) in 97patients, which was initiated on 1.4±0.8 days after surgery. They reported that in-hospital mortality was significantly lower in the high-dose group (62% vs. 82%). Unfortunately, in Japan, the UF volume in continuous RRT is restricted to 600 to 800 mL/h, according to the coverage of insurance.
RRT duration and weaning criteria
Predictors for successful weaning from RRT include urine output >400 mL/day without diuretics or >2,300 mL/day with diuretic use (35). However, it is unclear whether it is adequate to terminate simply the continuous RRT or whether weaning from RRT should be followed by intermittent RRT. In such a situation, the goal-directed RRT (GDRRT) for AKI after cardiac surgery by Xu et al. (36) may be useful. They advocated the following as a goal of RRT: (I) blood data: BUN ≤30 mmol/L, hematocrit ≥30%; (II) fluid balance: urine output ≥0.5 mL/kg/h with improved pulmonary congestion (an oxygen saturation ≥93%) and peripheral edema; (III) normalization of electrolytes and pH (3.5< K ≤5.5 mEq/L, 7.25≤ pH <7.45); and (IV) hemodynamics: mean arterial pressure ≥65 mmHg and central venous pressure ≥8–12 mmHg with no use of vasoconstrictors.
Management of fluid balance during CPB
Backgrounds
The CPB induces systemic inflammatory reaction syndrome (SIRS) with multifactorial pathophysiology (37,38). The early phase occurs as a result of hemodilution, non-pulsatile perfusion, blood contact with non-endothelial surfaces and the late phase is driven by ischemia-reperfusion injury, endotoxemia, coagulation disorders, and reactions to heparin/protamine. Specific genomic differences, age, and other preoperative factors influence the magnitude of SIRS.
SIRS causes fluid retention, edema, and organ dysfunction, resulting in increased mortality and morbidity. In order to alleviate the adverse CPB-induced SIRS, UF is frequently used during CPB to concentrate diluted blood concentration and to improve edema and cardiopulmonary function.
Methods of UF
There are three methods of UF, to remove excess moisture and inflammation-related factors, as follows:
- Conventional UF (CUF): as Magilligan et al. reported in 1984 (39), CUF filters the blood during rewarming on CPB to remove some of the extra fluid. Blood is taken from the patient through the venous line and after it is pumped through the oxygenator it is passed through an ultrafilter. The hemoconcentrated blood is returned to the arterial line.
- Modified UF (MUF): as Elliott et al. reported in 1993 (40), MUG filters the patient’s blood via the aortic cannula in situ after weaning from CPB. Some use the venous cannula and run the ultrafilter pump at 10 to 30 mL/ kg, with a vacuum on the ultrafilter for 15 to 20 min to remove 600 to 750 mL of fluid. In pediatric cardiac surgery, usefulness of MUF has been reported to be effective in hemoconcentration, improved postoperative respiratory and left heart function, and reduction of blood transfusion volume (41). Prolonged exposure to the CPB machine, lowered body temperature, and the risk of air transmission have also been pointed out as a disadvantage, although equivalent effects in adult cardiac surgery has been reported (42).
- DUF or zero-balance UF (Z-BUF): in the on-bypass UF, as shown in Figure 1, the fluid that is removed using a hemoconcentrator during CPB is replaced with crystalloid or dialysate to remove inflammatory mediators but avoid pump-balance problems (43). This simple method without extending CPB time may be reasonably effective but has not proved very useful (44).
DUF
In the DUF during CPB, a polysulfone membrane blood concentrator with a membrane area of 1.1 m2 is incorporated in the venous line of the CPB circuit parallel to the venous reservoir or between the reservoir and the oxygenator. The venous blood returned to the reservoir is filtered by a blood concentrator, and then replaced with. The blood flow rate of the filtration circuit is set at 300 to 400 mL/min and the suction pressure is set crystalloid or dialysate to −20 mmHg. To improve water removal efficiency, albumin, mannitol, and hydroxyethyl starch are administered to achieve blood osmotic pressure of 280 mOsm.
In our experience of DUF in 108 patients (male 61, female 47; 68±15 years old; 16% on chronic HD) undergoing cardiac surgery under CPB for 213±81 min, we used crystalloid or dialysate of 10,920±3,812 mL, resulting in/out balance of −969±1,837 mL and rate of no transfusion of 20%. We also observed significantly increased blood osmotic pressure (264±6 to 278±5 mOsm), hematocrit (24.5%±3.7% to 31.6%±3.4%), serum sodium (131±5 to 137±4 mEq/L).
Management of postoperative fluid overload and edema
Cardiac surgery under CPB causes hemodilution, increased capillary permeability, and increased extracellular fluid, resulting in a marked excess fluid overload. Perioperative infusion and blood transfusions also promote fluid retention into the third space. The fluid overload increases mortality and morbidity, associated with pulmonary edema with disturbed oxygenation and increased intra-abdominal pressure reducing renal blood flow. In addition, the renin-angiotensin-aldosterone system is activated, resulting in exacerbates fluid retention (45).
Although it has been considered desirable to sufficiently maintain the preload by aggressive fluid hydration and avoid dehydration due to excessive diuretic administration (46), it has been shown that excess fluids are a factor in the prognosis of AKI patients. Bouchard et al. (47) showed that the mortality rate in patients with severe AKI whose in/out balance was >10% of their body weight at admission was significantly higher than that in the control group. Also, in severely ill pediatric patients, a fluid excess of 10–20% is considered to be a threshold for poor prognosis (48).
Loop diuretics are commonly used to improve fluid excess and edema after CPB surgery. However, when the patient is resistant to them, blood purification or RRT with little influence on hemodynamics should be started promptly according to the indications shown in Table 2.
Clinical impacts of blood purification
There are a few studies of meta-analysis regarding clinical impacts of blood purification for the patients undergoing cardiac surgery.
Association between UF during CPB and postoperative AKI
As described above, UF during CPB may protect the kidney and avoid homologous blood transfusions by blood concentration, filtration, and the balancing of shifts in the electrolyte plasma concentration as potassium overload (49). In contrast, there is an opinion that the use of UF during CPB to remove excessive fluid is not renal protective and may even lead to kidney damage, setting a limit of the removed volume to 2,900 mL or 42 mL/kg. A recent meta-analysis found no significant difference in AKI incidence between patients undergoing UF and those who have not, and no increase in AKI incidence in studies that removed an UF volume >2,900 mL (50). Their subgroup analysis found no significant difference in AKI incidence between UF groups, MUF and CUF. They concluded that UF in cardiac surgery is safe and does not increase the risk of AKI, even in patients with previous kidney problems.
Association between UF during CPB and postoperative blood loss
Although a previous meta-analysis comparing MUF and CUF in pediatric cardiac patients demonstrated significantly higher post-CPB hematocrit and mean arterial blood pressures with MUF (51), there are a few meta-analysis studies focusing on the effects of MUF in adult cardiac patients. A recent meta-analysis including 13 randomized controlled trials comprising 626 patients in the MUF group and 610 patients in the control group reported a significantly improved postoperative hematocrit, lower chest tube drainage, lower postoperative blood transfusion rate and shorter duration of ICU stay in the MUF group, although there was no difference in ventilation time or mortality rates (52).
Early vs. late initiation of RRT after cardiac surgery
There are fewer studies that specifically focus on the timing of RRT in cardiac surgery patients, and conclusions are somewhat conflicting (52). A large contemporary meta-analysis including 1,479 cardiac surgery patients in 15 different studies reported that AKI treated with early RRT had decreased 28-day mortality, shortened ICU and hospital length of stay, and reduced duration of RRT (53). In contrast, another recent meta-analysis including 4 studies that enrolled 335 patients who received RRT for postoperative AKI reported no difference in mortality for those receiving early and late initiation of RRT. They also reported that early RRT did not reduce the length of ICU or hospital stay (54). There are several reasons for such controversial results. Postoperative AKI is not a specific syndrome, but is a complex pathophysiological process, depending on age, gender, cardiac condition, diabetes, use of catecholamines, anemia, and so on.
Conclusions
Blood purification or RRT plays an important role in perioperative management of cardiac surgery under highly invasive CPB to treat critically ill patients. The RTT modes include intermittent HD and CVVH, SLED, and UF in the CPB circuit. There should be common recognition about the up-to-date indications, management ways, and risks for the RTT among a wide range of professions, including cardiac surgeons, anesthesiologists, clinical engineers, ICU physicians, and nephrologists.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jxym.amegroups.com/article/view/10.21037/jxym-23-3/coif). Y Takami serves as an unpaid editorial board member of Journal of Xiangya Medicine from June 2022 to May 2024. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- de Mendonça-Filho HT, Pereira KC, Fontes M, et al. Circulating inflammatory mediators and organ dysfunction after cardiovascular surgery with cardiopulmonary bypass: a prospective observational study. Crit Care 2006;10:R46. [Crossref] [PubMed]
- Villa G, Neri M, Bellomo R, et al. Nomenclature for renal replacement therapy and blood purification techniques in critically ill patients: practical applications. Crit Care 2016;20:283. [Crossref] [PubMed]
- Essential points from Evidence-based Clinical Practice Guidelines for Chronic Kidney Disease 2018. Clin Exp Nephrol 2019;23:1-15. [Crossref] [PubMed]
- Nitta K, Abe M, Masakane I, et al. Annual dialysis data report 2018, JSDT Renal Data Registry: dialysis fluid quality, hemodialysis and hemodiafiltration, peritoneal dialysis, and diabetes. Ren Replace Ther 2020;6:51. [Crossref]
- Stenvinkel P, Heimbürger O, Lindholm B, et al. Are there two types of malnutrition in chronic renal failure? Evidence for relationships between malnutrition, inflammation and atherosclerosis (MIA syndrome). Nephrol Dial Transplant 2000;15:953-60. [Crossref] [PubMed]
- Franga DL, Kratz JM, Crumbley AJ, et al. Early and long-term results of coronary artery bypass grafting in dialysis patients. Ann Thorac Surg 2000;70:813-8; discussion 819. [Crossref] [PubMed]
- Otaki M, Enmoto T, Oku H. Coronary bypass grafting for patients dependent on dialysis: modified ultrafiltration for perioperative management. ASAIO J 2003;49:650-4. [Crossref] [PubMed]
- Takami Y, Tajima K, Okada N, et al. Simplified management of hemodialysis-dependent patients undergoing cardiac surgery. Ann Thorac Surg 2009;88:1515-9. [Crossref] [PubMed]
- Quiroga B, Verde E, Abad S, et al. Detection of patients at high risk for non-occlusive mesenteric ischemia in hemodialysis. J Surg Res 2013;180:51-5. [Crossref] [PubMed]
- Huen SC, Parikh CR. Predicting acute kidney injury after cardiac surgery: a systematic review. Ann Thorac Surg 2012;93:337-47. [Crossref] [PubMed]
- Bellomo R, Ronco C, Kellum JA, et al. Acute renal failure - definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care 2004;8:R204-12. [Crossref] [PubMed]
- Parolari A, Pesce LL, Pacini D, et al. Risk factors for perioperative acute kidney injury after adult cardiac surgery: role of perioperative management. Ann Thorac Surg 2012;93:584-91. [Crossref] [PubMed]
- Rahmanian PB, Kröner A, Langebartels G, et al. Impact of major non-cardiac complications on outcome following cardiac surgery procedures: logistic regression analysis in a very recent patient cohort. Interact Cardiovasc Thorac Surg 2013;17:319-26; discussion 326-7. [Crossref] [PubMed]
- Lopez-Delgado JC, Esteve F, Torrado H, et al. Influence of acute kidney injury on short- and long-term outcomes in patients undergoing cardiac surgery: risk factors and prognostic value of a modified RIFLE classification. Crit Care 2013;17:R293. [Crossref] [PubMed]
- Dardashti A, Ederoth P, Algotsson L, et al. Incidence, dynamics, and prognostic value of acute kidney injury for death after cardiac surgery. J Thorac Cardiovasc Surg 2014;147:800-7. [Crossref] [PubMed]
- Ng SY, Sanagou M, Wolfe R, et al. Prediction of acute kidney injury within 30 days of cardiac surgery. J Thorac Cardiovasc Surg 2014;147:1875-83, 1883.e1.
- Sato Y, Kato TS, Oishi A, et al. Preoperative factors associated with postoperative requirements of renal replacement therapy following cardiac surgery. Am J Cardiol 2015;116:294-300. [Crossref] [PubMed]
- Ortega-Loubon C, Fernández-Molina M, Fierro I, et al. Postoperative kidney oxygen saturation as a novel marker for acute kidney injury after adult cardiac surgery. J Thorac Cardiovasc Surg 2019;157:2340-2351.e3. [Crossref] [PubMed]
- KDIGO. KDIGO Clinical Practice Guideline for Acute Kidney Injury. Dialysis Interventions for Treatment of AKI. Kidney Int Suppl 2012;2:89-115.
- Gandhi A, Husain M, Salhiyyah K, et al. Does perioperative furosemide usage reduce the need for renal replacement therapy in cardiac surgery patients? Interact Cardiovasc Thorac Surg 2012;15:750-5. [Crossref] [PubMed]
- O'Neal JB, Shaw AD, Billings FT 4th. Acute kidney injury following cardiac surgery: current understanding and future directions. Crit Care 2016;20:187. [Crossref] [PubMed]
- Zarbock A, Kellum JA, Schmidt C, et al. Effect of Early vs Delayed Initiation of Renal Replacement Therapy on Mortality in Critically Ill Patients With Acute Kidney Injury: The ELAIN Randomized Clinical Trial. JAMA 2016;315:2190-9. [Crossref] [PubMed]
- Liu Y, Davari-Farid S, Arora P, et al. Early versus late initiation of renal replacement therapy in critically ill patients with acute kidney injury after cardiac surgery: a systematic review and meta-analysis. J Cardiothorac Vasc Anesth 2014;28:557-63. [Crossref] [PubMed]
- Leite TT, Macedo E, Pereira SM, et al. Timing of renal replacement therapy initiation by AKIN classification system. Crit Care 2013;17:R62. [Crossref] [PubMed]
- STARRT-AKI Investigators. Timing of Initiation of Renal-Replacement Therapy in Acute Kidney Injury. N Engl J Med 2020;383:240-51. [Crossref] [PubMed]
- Schefold JC, von Haehling S, Pschowski R, et al. The effect of continuous versus intermittent renal replacement therapy on the outcome of critically ill patients with acute renal failure (CONVINT): a prospective randomized controlled trial. Crit Care 2014;18:R11. [Crossref] [PubMed]
- Wang AY, Bellomo R. Renal replacement therapy in the ICU: intermittent hemodialysis, sustained low-efficiency dialysis or continuous renal replacement therapy? Curr Opin Crit Care 2018;24:437-42. [Crossref] [PubMed]
- Marshall MR, Golper TA, Shaver MJ, et al. Sustained low-efficiency dialysis for critically ill patients requiring renal replacement therapy. Kidney Int 2001;60:777-85. [Crossref] [PubMed]
- Wu VC, Wang CH, Wang WJ, et al. Sustained low-efficiency dialysis versus continuous veno-venous hemofiltration for postsurgical acute renal failure. Am J Surg 2010;199:466-76. [Crossref] [PubMed]
- Ronco C, Bellomo R, Homel P, et al. Effects of different doses in continuous veno-venous haemofiltration on outcomes of acute renal failure: a prospective randomised trial. Lancet 2000;356:26-30. [Crossref] [PubMed]
- VA/NIH Acute Renal Failure Trial Network. Intensity of renal support in critically ill patients with acute kidney injury. N Engl J Med 2008;359:7-20. [Crossref] [PubMed]
- RENAL Replacement Therapy Study Investigators. Intensity of continuous renal-replacement therapy in critically ill patients. N Engl J Med 2009;361:1627-38. [Crossref] [PubMed]
- Bent P, Tan HK, Bellomo R, et al. Early and intensive continuous hemofiltration for severe renal failure after cardiac surgery. Ann Thorac Surg 2001;71:832-7. [Crossref] [PubMed]
- Li SY, Yang WC, Chuang CL. Effect of early and intensive continuous venovenous hemofiltration on patients with cardiogenic shock and acute kidney injury after cardiac surgery. J Thorac Cardiovasc Surg 2014;148:1628-33. [Crossref] [PubMed]
- Uchino S, Bellomo R, Morimatsu H, et al. Discontinuation of continuous renal replacement therapy: a post hoc analysis of a prospective multicenter observational study. Crit Care Med 2009;37:2576-82. [Crossref] [PubMed]
- Xu J, Ding X, Fang Y, et al. New, goal-directed approach to renal replacement therapy improves acute kidney injury treatment after cardiac surgery. J Cardiothorac Surg 2014;9:103. [Crossref] [PubMed]
- Rubens FD, Mesana T. The inflammatory response to cardiopulmonary bypass: a therapeutic overview. Perfusion 2004;19:S5-12. [Crossref] [PubMed]
- Squiccimarro E, Stasi A, Lorusso R, et al. Narrative review of the systemic inflammatory reaction to cardiac surgery and cardiopulmonary bypass. Artif Organs 2022;46:568-77. [Crossref] [PubMed]
- Magilligan DJ Jr, Oyama C. Ultrafiltration during cardiopulmonary bypass: laboratory evaluation and initial clinical experience. Ann Thorac Surg 1984;37:33-9. [Crossref] [PubMed]
- Elliott MJ. Ultrafiltration and modified ultrafiltration in pediatric open heart operations. Ann Thorac Surg 1993;56:1518-22. [Crossref] [PubMed]
- Daggett CW, Lodge AJ, Scarborough JE, et al. Modified ultrafiltration versus conventional ultrafiltration: a randomized prospective study in neonatal piglets. J Thorac Cardiovasc Surg 1998;115:336-41; discussion 341-2. [Crossref] [PubMed]
- Luciani GB, Menon T, Vecchi B, et al. Modified ultrafiltration reduces morbidity after adult cardiac operations: a prospective, randomized clinical trial. Circulation 2001;104:I253-9. [Crossref] [PubMed]
- Fukumura F, Kado H, Imoto Y, et al. Usefulness of low-priming-volume cardiopulmonary bypass circuits and dilutional ultrafiltration in neonatal open-heart surgery. J Artif Organs 2004;7:9-12. [Crossref] [PubMed]
- Berdat PA, Eichenberger E, Ebell J, et al. Elimination of proinflammatory cytokines in pediatric cardiac surgery: analysis of ultrafiltration method and filter type. J Thorac Cardiovasc Surg 2004;127:1688-96. [Crossref] [PubMed]
- Shotan A, Dacca S, Shochat M, et al. Fluid overload contributing to heart failure. Nephrol Dial Transplant 2005;20:vii24-7. [Crossref] [PubMed]
- Abuelo JG. Normotensive ischemic acute renal failure. N Engl J Med 2007;357:797-805. [Crossref] [PubMed]
- Bouchard J, Soroko SB, Chertow GM, et al. Fluid accumulation, survival and recovery of kidney function in critically ill patients with acute kidney injury. Kidney Int 2009;76:422-7. [Crossref] [PubMed]
- Sutherland SM, Zappitelli M, Alexander SR, et al. Fluid overload and mortality in children receiving continuous renal replacement therapy: the prospective pediatric continuous renal replacement therapy registry. Am J Kidney Dis 2010;55:316-25. [Crossref] [PubMed]
- Long DM, Jenkins E, Griffith K. Perfusionist techniques of reducing acute kidney injury following cardiopulmonary bypass: an evidence-based review. Perfusion 2015;30:25-32. [Crossref] [PubMed]
- Kandil OA, Motawea KR, Darling E, et al. Ultrafiltration and cardiopulmonary bypass associated acute kidney injury: A systematic review and meta-analysis. Clin Cardiol 2021;44:1700-8. [Crossref] [PubMed]
- Kuratani N, Bunsangjaroen P, Srimueang T, et al. Modified versus conventional ultrafiltration in pediatric cardiac surgery: a meta-analysis of randomized controlled trials comparing clinical outcome parameters. J Thorac Cardiovasc Surg 2011;142:861-7. [Crossref] [PubMed]
- Low ZK, Gao F, Sin KYK, et al. Modified ultrafiltration reduces postoperative blood loss and transfusions in adult cardiac surgery: a meta-analysis of randomized controlled trials. Interact Cardiovasc Thorac Surg 2021;32:671-82. [Crossref] [PubMed]
- Zou H, Hong Q, Xu G. Early versus late initiation of renal replacement therapy impacts mortality in patients with acute kidney injury post cardiac surgery: a meta-analysis. Crit Care 2017;21:150. [Crossref] [PubMed]
- Cui J, Tang D, Chen Z, et al. Impact of Early versus Late Initiation of Renal Replacement Therapy in Patients with Cardiac Surgery-Associated Acute Kidney Injury: Meta-Analysis with Trial Sequential Analysis of Randomized Controlled Trials. Biomed Res Int 2018;2018:6942829. [Crossref] [PubMed]
Cite this article as: Takami Y, Yamashiro T, Takagi Y. Blood purification for perioperative management of cardiac surgery. J Xiangya Med 2023;8:11.


