
The relationship between neutrophil-to-lymphocyte ratio, myeloperoxidase, interleukin-2 and coronary slow flow phenomenon
Highlight box
Key findings
• NLR, IL-2 and MPO are associated with CSF, both can be used as promising indicators of CSF.
What is known and what is new?
• The incidence of CSF was between 1–7% which can course adverse cardiac events, inflammation plays a crucial role in the development of plaque in coronary artery disease.
• This study was to investigate the inflammation markers as the risk factors for pectoris patients with CSF, with a particular focus on the inflammatory response.
What is the implication, and what should change now?
• Elevated IL-2, MPO and MPO level were associated with severe inflammation and higher incidence of CSF phenomenon in patients with angina. The aggravated inflammatory response caused by IL-2, MPO and MPO in angina patients needs more attention.
Instruction
The coronary slow flow (CSF) phenomenon is an angiography phenomenon which is characterized by contrast agent passes slowly but no obvious stenosis (1). The definition of CSF was corrected thrombolysis in myocardial infarction frame count (CTFC) less than 27 (2). With the development of non-pharmacological therapy including percutaneous coronary intervention (PCI) and advancement of pharmacological therapy including antiplatelet statin, the rate of CSF has decreased (3). For all this, the incidence of CSF was between 1–7% which can course adverse cardiac events (4), such as coronary hypoperfusion, malignant arrythmia event thought myocardial infarction. Over 80% patients manifested typical angina at rest and admitted with acute coronary syndrome. Accurate identification and diagnosis of CSF is necessary to help clinicians quickly make appropriate treatment decisions for patients.
It is a complex and multifactorial phenomenal involving inflammation, distal embolization, ischemic reperfusion injury and endothelial edema. Although first proposed by Tambe et al. (1) in 1972, the mechanism responsible for the CSF phenomenon has not been clearly investigated. A detailed understanding of CSF mechanism may help improve treatment efficiency. Inflammation plays a crucial role in the development of plaque in coronary artery disease (CAD) (5). The principal pathophysiology of CAD is generally considered to be coronary atherosclerosis, an inflammatory disorder caused by the formation of plaque and subsequent obstruction of the coronary arteries. The white blood cell (WBC) count as a clinical marker of inflammation, while the neutrophil-to-lymphocyte ratio (NLR) is use for revealing the systemic balance of inflammatory disruptors and protective factors. Since NLR has been published that increased NLR is associated with major adverse cardiovascular event.
Studies have shown that increased NLR as an independent risk factor in different populations, such as ST-segment elevation myocardial infarction (STEMI) and non-STEMI (NSTEMI) patients with CSF (6,7). Some small studies have suggested that C-reactive protein (CRP) level, uric acid level, lymphocyte-to-monocyte ratio (LMR) are risk factors for CSF patients (8-10). There is evidence that interleukin and myeloperoxidase (MPO) are vital players in the chronic cardiovascular inflammation that is typical for atherosclerosis (11,12). To our knowledge, there is no consensus that the relationship between CSF phenomenon and interleukin-2 (IL-2), MPO, NLR in angina patients. Therefore, the aim of this study was to investigate the inflammation markers as the risk factors for pectoris patients with CSF, with a particular focus on the inflammatory response. We present the following article in accordance with the STROBE reporting checklist (available at https://jxym.amegroups.com/article/view/10.21037/jxym-22-47/rc).
Methods
Study population
This was a retrospectively observational study received from January 2018 to December 2021 at the Department of Cardiology, The Fifth Affiliated Hospital of Sun-Yat Seng University in Zhuhai, China. The patients enrolled in our study who were suspected of having CAD but coronary angiography (CAG) diagnosed with no significant coronary stenosis lesions. The exclusion criteria were as follows: history CAD, isolated coronary artery ectasia, acute coronary syndrome treatment with percutaneous coronary intervention or thrombolysis, chronic or acute heart failure, congenital heart disease, valuer heart disease, cardiomyopathy, acute inflammatory diseases with 1 month and connective tissue disease. A total of 100 continued individuals were included and grouped into the CSF group (n=50) and the non-CSF group (n=50) who were underwent CAG.
Angiography data and frame counting
CAG was performed by two experienced operators who used classic Judkins approach via radial or femoral route and were blinded all information of patients. Ionic- contrast low osmolality contrast medium was used in the procedure and the frames data were collected at a film rate of 30 frames per second. Our study determined using corrected thrombolysis in myocardial infarction (TIMI) frame count to assess CSF as described by Gibson et al. (2). In this method, the first frame and the final frame should be calculated, the counts were collected from contrast agent fulfill coronary artery to contrast reach the given distal landmark of certain coronary artery. Due to left anterior descending (LAD) artery longer than left circumflex (LCX), the TIMI frame count (TFC) should divide 1.7 to obtain CTFC (2). Three artery CTFC were summed and divided by 3 to obtain mean CTFC. Published reference frame counts are 36±2.6 for LAD, 20±3 for right coronary artery (RCA) and 22±4 for LCX. CSF phenomenon is diagnosed by a standard deviation greater than 2 declared normal coronary artery flow rates. Gibson et al. found that used the CTFC ≥27 frames as a cutoff value of CSF.
Data collection and definition
Age, sex, systolic blood pressure (SBP), diastolic blood pressure, smoking history, diabetes mellitus, hypertension history, body mass index (BMI), drug therapy history, blood count analyses, MPO and IL-2 were obtained at the time of admission. CTFC data was measured and recorded by two experienced operators. Diabetes mellitus was based on the presence of blood glucose concentrations equal to or greater than 7.0 mmol/L after an overnight fast or on the presence of blood glucose concentrations greater than 11.1 mmol/L in general. Hypertension was defined SBP ≥140 mmHg and (or) diastolic blood pressure ≥90 mmHg. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Ethical approval was obtained from The Fifth Affiliated Hospital of Sun Yat-sen University Ethics Committee before the commencement of the registry by participating institutions (2022 No. K75-1). Written informed consent was obtained from each subject enrolled in the study.
Statistical analysis
Statistical analysis was performed using the SPSS version 25.0 software (IBM Corp., Armonk, New York, USA). Continuous variables that conform to a normal distribution are expressed as means ± standard deviations, and those that do not conform are expressed as medians (25th, 75th percentiles). Categorical variables are expressed as absolute values and percentages. Kolmogorov-Smirnov test detected whether the variables conform to the normal distribution. Two sample independent t-test or Mann-Whitney U test was used to compare the continuous variables of two groups according to whether normally distributed. Comparing two groups of categorical variables were performed using chi-squared test. Spearman correlation analysis screens for risk factors associated with NLR and CSF. Backward stepwise univariate and multivariate logistic regression analysis were performed to screen independent risk factors for CSF. The receiver operating characteristic (ROC) curve determines the sensitivity and specificity of independent predictors of CSF
Results
Two groups of demographics and clinical characteristics were presented at Table 1. The proportion of male (64% vs. 40%, P=0.012), patients with smoking history (34% vs. 16%, P=0.032) and BMI (27.88±3.67 vs. 23.65±3.78 kg/m2, P=0.001) was significantly higher in the CSF group than in the non-CSF group. Besides, the CTFC of LAD (37.06±4.67 vs. 20.64±2.62, P<0.001), LCX (35.57±4.61 vs. 20.56±2.57, P<0.001) and RCA (33.02±4.26 vs. 20.72±2.54, P<0.001) were significantly higher in the CSF group than non-CSF group. However, there was no significant difference between two groups in terms of blood pressure, drug therapy and proportion of diabetes mellitus.
Table 1
| Characteristics | CSF (n=50) | Non-CSF (n=50) | P value |
|---|---|---|---|
| Clinical features | |||
| Age (years) | 56.67±6.64 | 55.48±7.74 | 0.401 |
| Gender (male/female) | 32/18 | 20/30 | 0.012 |
| SBP (mmHg) | 125.56±17.82 | 127.35±14.42 | 0.085 |
| DBP (mmHg) | 75.15±8.25 | 77.67±6.40 | 0.085 |
| Smoking history | 17 [34] | 8 [16] | 0.032 |
| Diabetes mellitus | 5 [10] | 6 [12] | 0.876 |
| Hypertension history | 8 [16] | 6 [12] | 0.537 |
| BMI (kg/m2) | 27.88±3.67 | 23.65±3.78 | 0.001 |
| Drug therapy | |||
| β-block | 5 [10] | 4 [8] | 0.703 |
| ACEI/ARB | 7 [14] | 6 [12] | 0.736 |
| CCB | 8 [16] | 7 [14] | 0.747 |
| Statins | 15 [30] | 14 [28] | 0.775 |
| CTFC | |||
| LAD CTFC | 37.06±4.67 | 20.64±2.62 | <0.001 |
| LCX CTFC | 35.57±4.61 | 20.56±2.57 | <0.001 |
| RCA CTFC | 33.02±4.26 | 20.72±2.54 | <0.001 |
Data were presented as mean ± SD or n [%]. CSF, coronary slow flow; SBP, systolic blood pressure; DBP, diastolic blood pressure; BMI, body mass index; ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blockers; CTFC, corrected thrombolysis in myocardial infarction frame count; LAD, left anterior descending coronary artery; LCX, left circumflex coronary artery; RCA, right coronary artery; SD, standard deviation.
The comparison result of laboratory examinations between two groups was presented at Table 2. The terms of CSF group, NLR [3.46 (2.52, 7.37) vs. 1.59 (1.26, 2.28), P<0.001], neutrophils counts (5.42±1.86 vs. 3.63±1.28, P<0.001), MPO [59.57 (49.65, 89.25) vs. 45.23 (34.19, 56.25), P<0.001], IL-2 [5.35 (2.95, 5.96) vs. 2.17 (1.94, 2.94), P<0.001] were significantly higher than non-CSF group, but lymphocytes was lower than non-CSF group. However, there was no significant difference in the triglyceride (TG), total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), WBC counts, high-sensitivity CRP and creatinine between two groups.
Table 2
| Characteristics | CSF (n=50) | Non-CSF (n=50) | P value |
|---|---|---|---|
| Laboratory examinations | |||
| TG (mmol/L) | 1.64±0.75 | 1.52±0.48 | 0.349 |
| TC (mmol/L) | 4.67±0.92 | 4.64±0.93 | 0.152 |
| HDL-C (mmol/L) | 1.03±0.17 | 1.04±0.14 | 0.616 |
| LDL-C (mmol/L) | 2.85±0.60 | 2.95±0.51 | 0.373 |
| NLR | 3.46 (2.52, 7.37) | 1.59 (1.26, 2.28) | <0.001 |
| LYM (109/L) | 1.42 (0.76, 1.84) | 2.07 (1.86, 2.60) | <0.001 |
| NEU (109/L) | 5.42±1.86 | 3.63±1.28 | <0.001 |
| WBC (109/L) | 6.73±2.27 | 6.86±1.89 | 0.828 |
| HsCRP (mg/L) | 2.41 (1.57, 4.82) | 1.92 (1.37, 3.32) | 0.057 |
| HGB (g/L) | 141.61±15.63 | 135.9±14.06 | 0.059 |
| CRE (μmmol/L) | 59.67±15.56 | 57.68±15.26 | 0.521 |
| MPO (ng/mL) | 59.57 (49.65, 89.25) | 45.23 (34.19, 56.25) | <0.001 |
| IL-2 | 5.35 (2.95, 5.96) | 2.17 (1.94, 2.94) | <0.001 |
| CTFC | |||
| LAD CTFC | 37.06±4.67 | 20.64±2.62 | <0.001 |
| LCX CTFC | 35.57±4.61 | 20.56±2.57 | <0.001 |
| RCA CTFC | 33.02±4.26 | 20.72±2.54 | <0.001 |
Data were presented as mean ± SD or medians (25th, 75th percentiles). CSF, coronary slow flow; TG, triglyceride; TC, total cholesterol; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; NLR, neutrophil-to-lymphocyte ratio; LYM, lymphocyte; NEU, neutrophil; WBC, white blood cell; HsCRP, high-sensitivity C-reactive protein; HGB, hemoglobin; CRE, creatinine; MPO, myeloperoxidase; IL-2, interleukin-2; CTFC, corrected thrombolysis in myocardial infarction frame count; LAD, left anterior descending coronary artery; LCX, left circumflex coronary artery; RCA, right coronary artery; SD, standard deviation.
Spearman correlation analysis was performed using to detect the risk factors between gender, smoking history, BMI, neutrophils count, lymphocytes count, IL-2, NLR, MPO and CSF. Accordingly, there was a significant and positive correlation between CSF and smoking history (r=0.215, P=0.032), BMI (r=0.498, P<0.001), neutrophils count (r=0.475, P<0.001), IL-2 (r=0.613, P<0.001), NLR (r=0.758, P<0.001), MPO (r=0.623, P<0.001), whereas the lymphocytes count had a negative and significant correlation with CSF. On the other hand, the male was found have a positive correlation with CSF but no statistically significant. The correlation analysis was presented at Table 3.
Table 3
| Variables | r | P value |
|---|---|---|
| Male | 0.061 | 0.546 |
| Smoking history | 0.215 | 0.032 |
| BMI | 0.498 | <0.001 |
| LYM | −0.479 | <0.001 |
| NEU | 0.475 | <0.001 |
| IL-2 | 0.613 | <0.001 |
| NLR | 0.758 | <0.001 |
| MPO | 0.623 | <0.001 |
CSF, coronary slow flow; BMI, body mass index; LYM, lymphocyte; NEU, neutrophil; IL-2, interleukin-2; NLR, neutrophil-to-lymphocyte ratio; MPO, myeloperoxidase.
Backward stepwise univariate and multivariate logistic regression analysis were performed to detect independent predictors of CSF. Univariable analysis revealed that male, BMI, lymphocytes, neutrophils, IL-2, NLR, MPO correlate with CSF. Whereas these parameters were analyzed by multivariable, only IL-2 [odds ratio (OR) 5.334 (95% confidence interval (CI): 1.314–21.737), P=0.019], NLR [OR 4.829 (95% CI: 1.235–8.889), P=0.024], MPO [OR 1.193 (95% CI: 1.031–1.381), P=0.018] were affirmed as independent predictors of CSF. These analyses were depicted at Table 4.
Table 4
| Variables | Univariate | Multivariate | |||
|---|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | ||
| Male | 3.144 (1.072–9.223) | 0.037 | 6.110 (0.136–9.121) | 0.706 | |
| BMI | 1.650 (1.274–2.137) | <0.001 | 1.596 (0.889–2.893) | 0.124 | |
| LYM | 0.180 (0.064–0.508) | 0.001 | 1.133 (0.036–36.046) | 0.943 | |
| NEU | 2.857 (1.662–4.912) | <0.001 | 1.508 (0.415–5.481) | 0.533 | |
| IL-2 | 2.930 (1.723–4.983) | <0.001 | 5.334 (1.314–21.737) | 0.019 | |
| NLR | 4.377 (1.863–9.286) | 0.001 | 4.829 (1.235–8.889) | 0.024 | |
| MPO | 1.112 (1.061–1.165) | <0.001 | 1.193 (1.031–1.381) | 0.018 | |
OR, odds ratio; CI, confidence interval; BMI, body mass index; LYM, lymphocyte; NEU, neutrophil; IL-2, interleukin-2; NLR, neutrophil-to-lymphocyte ratio; MPO, myeloperoxidase.
We used ROC curve to determine the sensitivity and specificity of independent predictors of slow coronary blood flow. The ROC curve revealed when an area under the curve (AUC) of 0.8726, using cutoff value of NLR ≥2.47 to predict CSF with sensitivity of 82% and specificity of 78%. The AUC value of IL-2 was 0.8514 with 92% sensitivity and 68% specificity at cutoff value above 3.345 pg/mL. The MPO was found to have AUC value of 0.8678 with an optimal cutoff value ≥46.74 ng/mL, the sensitivity and the specificity was 82% and 78% respectively. The ROC curve was shown at Figure 1.
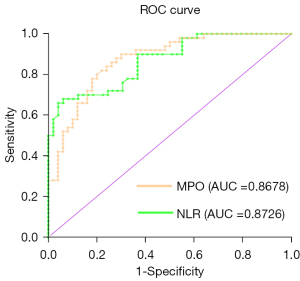
Discussion
In the present study, the pinpoint relationship between CSF patients and IL-2, MPO, NLR was discussed; our study showed that the NLR, IL-2, and MPO of CSF patients were higher and statistically significant compared to the non-CSF patients. In addition, NLR, IL-2, MPO were not only positively correlated with CSF, but also served as an independent predictor of CSF. The ROC curve revealed that these indicators could well distinguish between CSF and non-CSF patients with a high degree of specificity and sensitivity when the appropriate NLR, IL-2, MPO cut-off values were used.
The exact mechanism of CSF was still not well understood, and present literature suggest that it may be associated with inflammatory response, endothelial dysfunction, microvascular lesions, distal thrombosis, and ischemia-reperfusion injury. Previous studies evidence that the inflammatory response played a vital role in the occurrence and development of cardiovascular disease. For CVD patients, the inflammatory response was involved in the whole process of coronary plaque formation, rupture and embolism (13). Intravascular ultrasound (IVUS) technology was used for those CSF patients could also observe the diffuse intimal thickening and the calcified plaque deposition (14), which revealed that CSF as a subclinical atherosclerosis with normal CAG. Therefore, we hypothesis that various inflammatory factors may affect coronary heart disease also have the potential to influent and predict CSF phenomena.
NLR as a common marker of inflammation that could be easily and quickly obtained in clinical practice. Previous studies had described that in a variety of cardiovascular diseases patients were observed had a significant increase in NLR compared with control patients. Arbel et al. divided STEMI patients undergoing emergency PCI into low and high NLR groups, patients in the high NLR group had higher 30-day and 5-year mortality independently associated with lower LVEF (15). Verdoia et al. preferred that NLR is independently associated with the occurrence and the severity of CAD in a study of 3,738 patients with stable angina (16). CSF also as a cardiovascular event, hence we hypothesized that NLR was associated with CSF in our study. Through our investigation, further evidence that CSF was associated inflammation, not only that NLR can independently forecast CSF and CSF and non-CSF patients can be distinguished when selected the appropriate cut-off value. Consistent with previously published literature, Doğan et al. found that NLR was related to CSF and prognosticate that independently in a study of 82 patients with CSF phenomenal (17).
Inflammation was considered to be the main cause of atherosclerosis through adverse effects on lipoprotein metabolism and arterial walls (18). We hypothesized that CSF is a subclinical atherosclerotic, with IL-1b, IL-6, IL-2 as cytochromes secreted by type 1 CD4 T helper cells, which are considered pro-inflammatory factors and can aggravate atherosclerosis; Ørn et al. revealed that high levels of IL-1b and IL-6 were associated with a poor prognosis in patients with STEMI and served as a predictor of myocardial remodeling after myocardial infarction (19). Groot et al. followed up with IL-6 tests in STEMI patients undergoing PCI for 1 day to 4 months found that high IL-6 levels independently predicted a decline in left ventricular systolic function (20). In addition, Li et al. revealed a positive correlation between serum IL-6 levels and TFC (21). To our knowledge, there was no study show that whether the difference in serum IL-2 between CSF patients and non-CSF patients is statistically significant. Consequently, we performed our study to reveal IL-2 levels were positively correlated with CSF, and also can be used as an independent predictor of CSF, which is analogous to previous studies of other cytokines. So, we further demonstrated that CSF is a pre-atherosclerotic phenomenal and inflammation involved in the whole line of CSF.
MPO as an important peroxidase formed by activated neutrophil degranulation, which run through the entire process from early endothelial dysfunction to the formation of atherosclerotic plaque inflammatory response in the occurrence and development of cardiovascular disease (22). For patients with stable angina, MPO could produce a series of diffusible strong oxidants in vivo, such as hypochlorous acid, superoxide nitrite anions, active aldehydes and other oxidative modifications of LDL and HDL to promote atherosclerosis formation (23). For acute coronary syndrome, MPO is involved in the acute coronary syndromes (ACS) process by perturbation plaque stabilization. Zhang et al. performed a study with 2001 CAD patients revealed higher MPO levels than control patients (12). Ndrepepa et al. also revealed that MPO distinguished between ACS and non-ACS patients with ROC values of 0.731 (P<0.001) (24). Our research believed that CSF patients had higher MPO concentration than non-CSF patients, and as an independently predictor for CSF, appropriate cut-off values was selected to make a good distinction between CSF and non-CSF. This is consistent with Yurtdas et al. (25) investigation and other studies. MPO as an inflammation marker support a new entry point and theoretical basis for future diagnosis of CSF.
This study also has the following limitation: first, this study is a retrospective and observational study conducted in a single research center, with a small sample size. Second, most of the samples were collected at the time of hospitalization prior to angiography examination, and the decision to perform angiography examination depended on different operators, which may not exclude potential selection bias. So, more patients are needed in future studies.
Conclusions
In this study, elevated IL-2, MPO and NLR level were associated with severe inflammation and higher incidence of CSF phenomenon in patients with angina. The aggravated inflammatory response caused by IL-2, MPO and NLR in angina patients needs more attention.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jxym.amegroups.com/article/view/10.21037/jxym-22-47/rc
Data Sharing Statement: Available at https://jxym.amegroups.com/article/view/10.21037/jxym-22-47/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jxym.amegroups.com/article/view/10.21037/jxym-22-47/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Ethical approval was obtained from The Fifth Affiliated Hospital of Sun Yat-sen University Ethics Committee before the commencement of the registry by participating institutions (2022 No. K75-1). Written informed consent was obtained from each subject enrolled in the study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Tambe AA, Demany MA, Zimmerman HA, et al. Angina pectoris and slow flow velocity of dye in coronary arteries--a new angiographic finding. Am Heart J 1972;84:66-71. [Crossref] [PubMed]
- Gibson CM, Cannon CP, Daley WL, et al. TIMI frame count: a quantitative method of assessing coronary artery flow. Circulation 1996;93:879-88. [Crossref] [PubMed]
- Allencherril J, Jneid H, Atar D, et al. Pathophysiology, Diagnosis, and Management of the No-Reflow Phenomenon. Cardiovasc Drugs Ther 2019;33:589-97. [Crossref] [PubMed]
- Wang X, Nie SP. The coronary slow flow phenomenon: characteristics, mechanisms and implications. Cardiovasc Diagn Ther 2011;1:37-43. [PubMed]
- Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med 2005;352:1685-95. [Crossref] [PubMed]
- Hartopo AB, Puspitawati I, Setianto BY. On-admission High Neutrophil to Lymphocyte Ratio as Predictor of In-hospital Adverse Cardiac Event in ST-elevation Myocardial Infarction. Acta Med Indones 2015;47:3-10. [PubMed]
- Zengin A, Karaca M, Aruğaslan E, et al. Performance of neutrophil to lymphocyte ratio for the prediction of long-term morbidity and mortality in coronary slow flow phenomenon patients presented with non-ST segment elevation acute coronary syndrome. J Cardiovasc Thorac Res 2021;13:125-30. [Crossref] [PubMed]
- Niu H, Wei Z, Zhang Y, et al. Atorvastatin improves coronary flow and endothelial function in patients with coronary slow flow. Exp Ther Med 2018;15:904-8. [PubMed]
- Hu X, Yang X, Li X, et al. Elevated uric acid is related to the no-/slow-reflow phenomenon in STEMI undergoing primary PCI. Eur J Clin Invest 2022;52:e13719. [Crossref] [PubMed]
- Yang Z, Yuan J, Cui J, et al. Association of the lymphocyte-to-monocyte ratio, mean diameter of coronary arteries, and uric acid level with coronary slow flow in isolated coronary artery ectasia. BMC Cardiovasc Disord 2021;21:156. [Crossref] [PubMed]
- Mangge H, Hubmann H, Pilz S, et al. Beyond cholesterol--inflammatory cytokines, the key mediators in atherosclerosis. Clin Chem Lab Med 2004;42:467-74. [Crossref] [PubMed]
- Zhang R, Brennan ML, Fu X, et al. Association between myeloperoxidase levels and risk of coronary artery disease. JAMA 2001;286:2136-42. [Crossref] [PubMed]
- Niccoli G, Montone RA, Di Vito L, et al. Plaque rupture and intact fibrous cap assessed by optical coherence tomography portend different outcomes in patients with acute coronary syndrome. Eur Heart J 2015;36:1377-84. [Crossref] [PubMed]
- Cin VG, Pekdemir H, Camsar A, et al. Diffuse intimal thickening of coronary arteries in slow coronary flow. Jpn Heart J 2003;44:907-19. [Crossref] [PubMed]
- Arbel Y, Finkelstein A, Halkin A, et al. Neutrophil/lymphocyte ratio is related to the severity of coronary artery disease and clinical outcome in patients undergoing angiography. Atherosclerosis 2012;225:456-60. [Crossref] [PubMed]
- Verdoia M, Barbieri L, Di Giovine G, et al. Neutrophil to Lymphocyte Ratio and the Extent of Coronary Artery Disease: Results From a Large Cohort Study. Angiology 2016;67:75-82. [Crossref] [PubMed]
- Doğan M, Akyel A, Çimen T, et al. Relationship between neutrophil to lymphocyte ratio and slow coronary flow. Clin Appl Thromb Hemost 2015;21:251-4. [Crossref] [PubMed]
- Libby P, Ridker PM, Maseri A. Inflammation and atherosclerosis. Circulation 2002;105:1135-43. [Crossref] [PubMed]
- Ørn S, Ueland T, Manhenke C, et al. Increased interleukin-1β levels are associated with left ventricular hypertrophy and remodelling following acute ST segment elevation myocardial infarction treated by primary percutaneous coronary intervention. J Intern Med 2012;272:267-76. [Crossref] [PubMed]
- Groot HE, Al Ali L, van der Horst ICC, et al. Plasma interleukin 6 levels are associated with cardiac function after ST-elevation myocardial infarction. Clin Res Cardiol 2019;108:612-21. [Crossref] [PubMed]
- Li JJ, Qin XW, Li ZC, et al. Increased plasma C-reactive protein and interleukin-6 concentrations in patients with slow coronary flow. Clin Chim Acta 2007;385:43-7. [Crossref] [PubMed]
- Teng N, Maghzal GJ, Talib J, et al. The roles of myeloperoxidase in coronary artery disease and its potential implication in plaque rupture. Redox Rep 2017;22:51-73. [Crossref] [PubMed]
- Albert CJ, Crowley JR, Hsu FF, et al. Reactive chlorinating species produced by myeloperoxidase target the vinyl ether bond of plasmalogens: identification of 2-chlorohexadecanal. J Biol Chem 2001;276:23733-41. [Crossref] [PubMed]
- Ndrepepa G, Braun S, Mehilli J, et al. Myeloperoxidase level in patients with stable coronary artery disease and acute coronary syndromes. Eur J Clin Invest 2008;38:90-6. [Crossref] [PubMed]
- Yurtdaş M, Yaylali YT, Kaya Y, et al. Increased plasma high-sensitivity C-reactive protein and myeloperoxidase levels may predict ischemia during myocardial perfusion imaging in slow coronary flow. Arch Med Res 2014;45:63-9. [Crossref] [PubMed]
Cite this article as: Wei X, Zou Z, Chen B, Deng X, Yang G, Liao H. The relationship between neutrophil-to-lymphocyte ratio, myeloperoxidase, interleukin-2 and coronary slow flow phenomenon. J Xiangya Med 2023;8:3.


