
A multitask approach to prevention of the cognitive decline after coronary artery bypass grafting: a prospective randomized controlled study
Highlight box
Key findings
• The patients with multi-domains cognitive-motor training demonstrated lower frequency of a decrease in the indicators of executive functions, attention and short-term memory in the early postoperative period of coronary artery bypass grafting (CABG).
• The multi-domains cognitive-motor training resulted in a lower increase in slow-wave theta activity in the early postoperative period of CABG.
What is known and what is new?
• There is a great need for cognitive prevention in on-pump cardiac surgery patients due to high risk of brain ischemia and postoperative cognitive decline.
• The use of the multi-domains cognitive-motor training reduced the severity of postoperative cognitive decline in the early postoperative period of CABG.
What is the implication, and what should change now?
• The findings are encouraging from the point of view of the rehabilitation capacity of multi-domains cognitive-motor training.
• Further studies are needed to assess the longevity of the results of cognitive training.
Introduction
Coronary artery bypass grafting (CABG) remains one of the most common types of cardiac surgery. However, on-pump cardiac surgery is associated with high risk of brain ischemia and cognitive dysfunction. Postoperative cognitive dysfunction (POCD) occurs up to 70% of cases in the surgery patients, depending on the tactics of surgical intervention and diagnostic criteria (1,2). The associated with cardiopulmonary bypass and anesthesia memory and attention deficit can persist for a long time after surgery (3). Moreover, POCD contributes to a lower adherence to treatment, which in turn leads to the progression of the disease (4).
Recent literature highlights the need for extensive interdisciplinary study of postoperative cognitive decline, including neuropsychological tests, but also neuroimaging methods (5). The alteration in brain activity associated with coronary artery surgery can be studied using multi-channel electroencephalogram. Electroencephalography (EEG) is the non-invasive measurement of the neural electrical activity and is assumed to be one of the mechanisms of encoding and transmitting information (6,7). This assumption is based on the significant associations between specific frequency-spatial characteristics of brain oscillations and different cognitive functions (8), as well as the EEG changes associated with different brain pathologies (9,10). Previously, we also provided evidence on the use of EEG theta power to characterize brain dysfunction in cardiac patients (2,11).
There is currently a growing interest in research on the methods to prevent negative changes of the mental health of cardiac patients. Physical activity improves the cognitive abilities and mobility of the nervous system, can prevent cognitive disorders (12,13). However, patients in cardiac surgery generally have a lower functional reserve and a risk of complications in the early postoperative period. It is therefore difficult to choose a preventive strategy for postoperative cognitive decline because of the need to take into account the physical state of patients in cardiac surgery. Cognitive training has demonstrated its effectiveness in elderly people with cognitive impairment, including the patients after cardiac surgery (14-16). Computerized cognitive training is a low-cost, low-risk cognitive rehabilitation method that can be modified to treat both single- and multiple-domain cognitive impairments affecting different brain regions (17). The cognitive training sessions involve interaction with highly skilled specialists, including psychologists and neurologists and can continue for several weeks (18). There are different kinds of cognitive training programs that involve the simultaneous execution of several cognitive tasks, for example, internal counting, tasks that improve memory and attention, or divergent tasks in combination with motor tasks (walking, maintaining a certain posture or hand movement). It has been previously demonstrated that the performing both cognitive and motor tasks lead to a better recovery of memory and attention in elderly patients compared to motor or cognitive training performed separately (19).
Recent studies have established that the dual-task motor-cognitive training is a promising approach to prevention of cognitive disorders (19,20). The dual-task training is the simultaneous performance of motor (walking, running, maintenance of posture) and cognitive (working memory and attention, divergent thinking, backward counting) tasks. The simultaneous performance of different tasks is a key aspect of daily activity; therefore, choosing a multitasking approach is appropriate. Reduced ability to solve two or more tasks at once affects the quality of life of postoperative patients (21,22). Therefore, a multitask approach in cardiac surgery patients should include tasks corresponding to their physical condition in the postoperative period. As well, the level of complexity of cognitive tasks can gradually increase in the learning process. Previous studies have shown that dual-task programs have a beneficial effect on cognitive-motor functions in the elderly (23,24), in patients with traumatic brain injury (25), and in patients with Parkinson`s disease (26,27). However, no such studies have been conducted in a sample of cardiac surgery patients. In addition, the development of POCD in the early postoperative period is associated with long-term cognitive decline, making it necessary to begin cognitive training as soon as possible (28).
The necessity to objectively track the effectiveness of the training is the reason for the inclusion of monitoring of neurophysiological parameters in the examination of cardiac patients. Modern technologies of the human brain mapping, such as digital EEG, make it possible to follow changes in cognitive functions in cardiac patients (29). Taking it into account, the study aimed to assess the neurophysiological parameters (EEG and cognitive indicators) in on-pump CABG patients with and without the multi-domains cognitive-motor training. We present the following article in accordance with the CONSORT reporting checklist (available at https://jxym.amegroups.com/article/view/10.21037/jxym-22-37/rc).
Methods
Data collection and sampling
The patients with stable coronary artery disease (CAD) were chosen from the cohort of patients who underwent on-pump cardiac surgery at the clinic of the Research Institute for Complex Issues of Cardiovascular Diseases. The present study was in compliance with the Declaration of Helsinki (as revised in 2013) and approved by the Ethics Committee of Federal State Budgetary Institution of Research Institute of Complex Issues of Cardiovascular Diseases (protocol No. 10 dated 10/12/2020). The start of patient data collection was in January 2021. The inclusion criteria were as follows: stable CAD, elective CABG, aged 45–75 years, provided informed consent. The exclusion criteria were: history of stroke, epilepsy, traumatic brain injury, depression, dementia, Montreal Cognitive Assessment Scale (MoCA) score ≤18 (30), Beck’s Depression Inventory (BDI-II) score ≥8 (31), non-cardiovascular decompensated comorbidities. Based on a P value of 0.05, a study power of 80%, a group of 70 subjects was defined as a sample size. Five subjects have dropped-out due to coronavirus disease 2019 (COVID-19) diagnosed at the preoperative stage. Three patients have withdrawn from the study. Sixty-two patients were included in the study. Informed consent was taken from all the patients.
At the preoperative stage, all patients underwent neurological examination, cognitive and depression screening. Reviewers were blinded regarding the participation of patients in the study. The median age was 64 [60; 72] years. Most of the patients had a secondary special education, the median score according to the MoCA was 24, which indicates the presence of mild cognitive impairment. Depressive disorders were absent according to the BDI-II. Most of the patients were diagnosed with heart failure (HF) [functional class (FC) I–II] with a preserved left ventricular ejection fraction according to the classification of the Heart Failure Specialists Society [2002]. The history of CAD and arterial hypertension was more than five years (Table 1).
Table 1
| Characteristics | Patients with cognitive training (n=30) | Patients without training (n=32) | P value |
|---|---|---|---|
| Age (years), median [Q1; Q3] | 64.0 [60.0; 71.0] | 65.0 [61.0; 72.0] | 0.7 |
| History of CAD (years), median [Q1; Q3] | 5.0 [1.0; 7.0] | 5.0 [1.0; 10.0] | 0.5 |
| History of AH (years), median [Q1; Q3] | 12.0 [3.0; 15.0] | 13.0 [3.0; 15.0] | 0.3 |
| Heart failure, n (%) | 0.8 | ||
| 0 FC | 1 (3.3) | 0 (0.0) | |
| I–II FC | 25 (83.3) | 29 (90.6) | |
| III FC | 4 (13.3) | 3 (9.4) | |
| LVEF (%), median [Q1; Q3] | 62.0 [56.0; 66.0] | 61.0 [58.0; 65.0] | 0.8 |
| Type 2 diabetes mellitus, n (%) | 7 (23.3) | 6 (18.8) | 0.4 |
| Education (years), median [Q1; Q3] | 11.0 [10.0; 12.0] | 11.0 [10.0; 12.0] | 0.7 |
| MoCA score, median [Q1; Q3] | 24.0 [22.0; 27.0] | 23.0 [21.0; 28.0] | 0.4 |
| Beck’s Depression Inventory score, median [Q1; Q3] | 3.0 [2.0; 5.0] | 3.0 [1.0; 6.0] | 0.7 |
Q1, 25th percentile; Q3, 75th percentile; CAD, coronary artery disease; AH, arterial hypertension; FC, functional class; LVEF, left ventriculi ejection fraction; MoCA, Montreal Cognitive Assessment Scale.
A pseudo-randomization method was used to form two groups, comparable in terms of clinical characteristics. The main group consisted of 30 patients who received the cognitive training after CABG, in addition to standard postoperative management. The group without training included 32 patients. The overview of the study design can see on Figure 1.
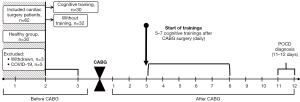
Elective on-pump CABG with normothermia was performed. Standard protocols for combined endotracheal anesthesia and perfusion were used. Intraoperative online monitoring of regional cerebral cortex oxygenation (rSO2) (INVOS-3100, Somanetics, Troy, MI, USA) was performed. Oxygen saturation indicators were within the normal range. The mean duration of cardiopulmonary bypass and aortic cross-clamping seen at the Table 2. Following CABG, all patients were transferred to the intensive care unit (ICU) for 1–2 days. After intensive care, they were transferred to the cardiology department for postoperative therapy and discharged in satisfactory condition for 11–12 days.
Table 2
| Characteristics | Patients with cognitive training (n=30), median [Q1; Q3] | Patients without training (n=32), median [Q1; Q3] | P value |
|---|---|---|---|
| Number of grafts | 3 [2; 3] | 3 [2; 3] | 0.8 |
| CPB duration (min) | 77.0 [60.0; 94.0] | 72.0 [66.0; 83.0] | 0.4 |
| Aortic cross-clamping (min) | 45.0 [35.0;55.0] | 42.0 [31.0; 50.0] | 0.3 |
| Perfusion temperature (℃) | 35.7 [35.5; 35.7] | 35.6 [35.4; 35.6] | 0.6 |
| Surgery duration (min) | 180.0 [160.0; 210.0] | 190.0 [165.0; 220.0] | 0.1 |
| Arterial blood pressure (mmHg) | 63.0 [62.0; 67.0] | 64.0 [61.0; 69.0] | 0.6 |
| Arterial oxygen saturation (%) | 99.1 [98.7; 99.0] | 99.0 [98.6; 99.2] | 0.5 |
Q1, 25th percentile; Q3, 75th percentile; CPB, cardiopulmonary bypass.
As a control group, healthy persons of the same age were enrolled in the study. This group included 30 people who had no signs of angina pectoris according to the Rose questionnaire [World Health Organization (WHO), 1984] (32) and whose score was equal to 6.0 [4.0; 8.0] in the questionnaire on the self-esteem of health by VP Voytenko (33).
Neuropsychological and neurophysiological examination
Cognitive status assessment
Prior to inclusion in the study, the cognitive status of the both groups of patients and control group assessed by the screening MoCA in validated Russian-language modified version. All participants of the study were also examined using extended neuropsychological testing (the assessment of psychomotor and executive function, attention, and short-term memory from the neuropsychological test battery of the software “Status PF” (34). A control group had a single neuropsychological examination. Cognitive indicators of this group were used as reference values for the patients with and without the multi-domains cognitive-motor training. The groups of patients with and without cognitive training underwent the extended neuropsychological testing 2–3 days before surgery and at 11–12 days after CABG. Alternate versions of the neuropsychological tests were used in repeated measurements to minimize practice effects. The postoperative cognitive decline was determined for each patient individually percentage of relative changes in postoperative indicators compared with baseline using the following formula: [(baseline value – postoperative value)/baseline value] ×100%. The negative values indicated an increase in the cognitive indicator compared to the baseline, positive values indicated a decrease, the threshold value for cognitive decline was equal to 20%.
Magnetic resonance imaging (MRI)
The patients with and without training underwent MRI 2–3 days before surgery to assess a baseline brain state. Neuroimaging was conducted using a 1.5 T Exelart Atlas MRI system (Toshiba, Japan). The width of the III ventricle was measured, the presence of leukoaraiosis, cysts, gliosis, enlargement of cerebrospinal fluid spaces was noted, and the Evans Index (EI) was measured as the linear ratio of the total width of the frontal horns of the cerebral lateral ventricles to the maximum intracranial diameter (normal EI for persons under 60 years: 24.0–26.3%; over 60 years: 28.2–29.4%) (35).
EEG recording and processing
Monopolar EEG recordings (0.1–50.0 Hz) were acquired using the international 10-10 placement system with 62 channels at rest, in a sitting position with closed eyes. The recording duration was 5 min. A SynAmps2 RT amplifier (Compumedics Neuroscan USA, Ltd. Charlotte, NC, USA) and a modified 64-channel electrode cap (QuikCap; Neurosoft, El Paso, TX, USA) were used. The reference electrode was placed at the tip of the nose, and the ground electrode was placed at the center of the forehead (resistance <5 kOhm). The data was analyzed offline, and a search for artifacts was carried out. The artifact-free EEG 2-second epochs were subjected to Fourier transform. Individual peak alpha frequency in the dual-task group averaged 9.35 Hz, therefore, the authors used typical frequency bands to average the values of EEG. This study involves the analysis of data of the theta-1 power (4–6 Hz), since the informational value of this frequency band for the diagnosis of vascular cognitive disorders was proved in the previous studies (2,11). The EEG values recorded with electrodes were summed up in five regions in the left and right hemispheres:
- Frontal (Fp1/2 + AF3/4 + F1/2 + Fp3/4 + Fp5/6 + F7/8);
- Central (FC1/2 + FC3/4 + FC5/6 + C1/2 + C3/4 + C5/6);
- Temporal (FT7/8 + T7/8 + TP7/8);
- Parietal (TP1/2 + TP3/4 + TP5/6 + P1/2 + P3/4 + P5/6 + P7/8);
- Occipital (PO3/4 + PO5/6 + PO7/8 + O1/2).
The parameters recorded on the midline (Fpz, Fz, etc.) were excluded from the analysis.
Multi-domains cognitive-motor training
A cognitive training course was started at 3–4 days after CABG, once daily for a period of 5–7 days. The daily training session lasted 20 min, was performed in the morning, and included a preparatory (2 min) and main (10–15 min) period. The duration of the training phase could be reduced at the patient’s request. The preparatory stage was involved a discussion with a neurologist. On the main stage, the patient performed a simple motor task and several verbal tasks: naming objects that start with a certain letter (letters may be changed upon moving to the next stage of the procedure), backward counting (sequential subtraction of 7 from 100) and solving a divergent task—“how to use unusually the usual item”, for example, a brick, a newspaper, or a plastic bottle.
Statistical analysis
Data analysis was conducted using the Statistica 10.0 software (StatSoft, Tulsa, OK, USA, SN: BXXR210F562022FA-A). Descriptive statistical methods were used for the analysis of the clinical and demographic parameters, the results are presented as median with interquartile range (IQR) [25th; 75th percentile] and the number of observations (n, %). The normality of the distribution of data was tested using the Shapiro-Wilk test. Most of the clinical and cognitive indicators were not normally distributed. Two-tailed Mann-Whitney U and Wilcoxon tests were used for continuous variables. Continuity-corrected χ2 were used for categorical variables and percentage of relative change in postoperative indicators. The differences were considered statistically significant at P<0.05. The EEG data were normalized using log transformation, and further analysis was carried out using repeated measures analysis of variance (ANOVA).
Results
Preoperative neurophysiological status of patients
MRI data
Neuroimaging features of cerebral small vessel disease (35) such as leukoaraiosis, enlargement of cerebrospinal fluid spaces was noted in the majority of patients; there were no differences between the groups of patients (Table 3).
Table 3
| Characteristics | Patients with cognitive training (n=30) | Patients without training (n=32) | P value |
|---|---|---|---|
| The width of the third ventricle (mm), median [Q1; Q3] | 7.0 [6.0; 8.0] | 7.0 [6.0; 8.0] | 0.2 |
| Cysts/gliosis, n (%) | 3 (10.0) | 5 (15.6) | 0.3 |
| Leukoaraiosis, n (%) | 22 (73.3) | 23 (71.9) | 0.8 |
| Evans Index (%), median [Q1; Q3] | 30.2 [26.5; 34.0] | 30.5 [26.0; 34.0] | 0.8 |
| Signs of cortical atrophy, n (%) | 5 (16.7) | 7 (21.9) | 0.5 |
| Enlargement of perivascular spaces, n (%) | 27 (90.0) | 29 (90.6) | 0.8 |
| Enlargement of subarachnoid space, n (%) | 28 (93.3) | 29 (90.6) | 0.8 |
Q1, 25th percentile; Q3, 75th percentile.
Neuropsychological indicators
Before surgery, the psychomotor and executive function, attention, and short-term memory were analyzed, and there are no significant between-group differences. However, as compared to a group of healthy individuals of the same age, the cognitive parameters of the patients with and without cognitive training were worse: a reaction time was significantly longer and they made more mistakes and missed more signals in executive function tests (Table 4). Similar differences were observed with short-term memory and attention indicators. Performing the Bourdon test, patients processed a fewer number of symbols both on the 1st and on the 4th minute of the test, indicating worse workability and attention fatigue.
Table 4
| Indicators | Groups, median [Q1; Q3] | P value | |||||
|---|---|---|---|---|---|---|---|
| Healthy participants [1] | Cognitive training [2] | Without training [3] | [1] vs. [2] | [1] vs. [3] | [2] vs. [3] | ||
| Brain responses to “feedback” | |||||||
| RT (ms) | 384 [378; 427] | 455 [412; 482] | 441 [408; 473] | <0.0001 | <0.0001 | 0.4 | |
| Errors (n) | 144 [126; 150] | 101 [89; 128] | 107 [83; 122] | 0.0006 | 0.0002 | 0.9 | |
| Level of functional mobility of nervous processes responses to “feedback” | |||||||
| RT (ms) | 411 [401; 428] | 477 [467; 495] | 473 [449; 504] | <0.0001 | <0.0001 | 0.6 | |
| Errors (n) | 25 [22; 29] | 23 [20; 28] | 25 [23; 27] | 0.5 | 0.3 | 0.4 | |
| MS (n) | 14 [5; 19] | 19 [12; 23] | 16 [11; 23] | 0.05 | 0.1 | 0.6 | |
| Memory | |||||||
| Numbers (n) | 6 [6; 6] | 4 [3; 5] | 5 [ 4; 5] | 0.03 | 0.04 | 0.2 | |
| Syllables (n) | 4 [3; 4] | 2 [2; 3] | 2 [2; 3] | 0.02 | 0.0008 | 0.8 | |
| Words (n) | 6 [4; 6] | 4 [3; 5] | 4 [3; 5] | 0.0006 | 0.001 | 0.7 | |
| The Bourdon’s test | |||||||
| Errors (n) | 8 [6; 16] | 8 [4; 11] | 10 [4; 14] | 0.3 | 0.3 | 0.4 | |
| 1st min (symbols n) | 127 [110; 142] | 64 [50; 76] | 72 [47; 102] | 0.001 | <0.0001 | 0.3 | |
| 4th min (symbols n) | 125 [112; 130] | 100 [76; 127] | 100 [65; 118] | 0.0002 | 0.0002 | 0.5 | |
Q1, 25th percentile; Q3, 75th percentile; RT, reaction time; MS, missed signals; Words, 10 words memorizing test; Numbers, 10 numbers memorizing test; Syllables, 10 nonsense syllable memorizing test; 1st min, processed symbols in the 1st min; 4th min, processed symbols in 4th min.
EEG indicators
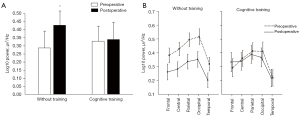
A repeated-measured ANOVA with a between-subjects factor Group (2 levels: with and without cognitive training) and within-subjects factors of Area (5 levels: frontal, central, parietal, occipital and temporal) and Laterality (2 levels: left/right hemisphere) was carried out. There were no between-group differences in baseline values of theta-1 power. The significance of the factors shared by the two groups was established: Area [F(4, 240)=33.31, P≤0.0001], as well as the interaction Area × Laterality [F(4, 240)=4.34, P=0.009]. Using the method of planned comparisons, it was found that the higher values of theta power were observed in the posterior cortex compared to the frontal cortex (frontal-occipital gradient) [F(1, 60)=41.86, P≤0.0001]. The left hemisphere, compared with the right, shows higher indicators of band power in the temporal cortex [F(1, 60)=6.42, P=0.01].
Postoperative changes in the neurophysiological status of patients
In the early postoperative period, significant adverse cardiovascular events did not occur in two groups of patients (stroke, heart attacks, cardiac arrhythmias, repeated unplanned revascularization).
Neuropsychological indicators
The analysis of the results of extended neuropsychological testing at 11–12 days after CABG revealed that the patients with and without cognitive training had no differences in the postoperative indicators of psychomotor and executive functions, memory and attention. However, all the patients were also worse compared to the group of healthy individuals (Table 5).
Table 5
| Indicators | Groups, median [Q1; Q3] | P value | |||||
|---|---|---|---|---|---|---|---|
| Healthy participants [1] | Cognitive training [2] | Without training [3] | [1] vs. [2] | [1] vs. [3] | [2] vs. [3] | ||
| Brain responses to “feedback” | |||||||
| RT (ms) | 384 [378; 427] | 446 [417; 527] | 451 [404; 492] | <0.0001 | <0.0001 | 0.5 | |
| Errors (n) | 144 [126; 150] | 105 [86; 138] | 106 [98; 124] | 0.007 | 0.0005 | 0.9 | |
| MS (n) | 59 [26; 81] | 61 [35; 80] | 68 [40; 75] | 0.9 | 0.7 | 0.3 | |
| Level of functional mobility of nervous processes responses to “feedback” | |||||||
| RT, ms | 411 [401; 428] | 485 [450; 522] | 474 [436; 546] | <0.0001 | <0.0001 | 0.9 | |
| Errors (n) | 25 [22; 29] | 27 [23; 30] | 26 [22; 29] | 0.09 | 0.2 | 0.6 | |
| MS (n) | 14 [5; 19] | 15 [11; 20] | 16 [13; 20] | 0.5 | 0.3 | 0.5 | |
| Memory | |||||||
| Numbers (n) | 6 [6; 6] | 4 [3; 5] | 5 [ 3; 5] | 0.001 | 0.6 | 0.08 | |
| Syllables (n) | 4 [3; 4] | 3 [2; 3] | 2 [1; 3] | 0.004 | 0.001 | 0.3 | |
| The words (n) | 6 [4; 6] | 4 [3; 5] | 4 [4; 5] | 0.0003 | 0.008 | 0.2 | |
| The Bourdon’s test | |||||||
| Errors (n) | 8 [6; 16] | 8 [5; 13] | 8 [4; 13] | 0.1 | 0.2 | 0.7 | |
| 1st min (symbols n) | 127 [110; 142] | 66 [45; 99] | 77 [51; 106] | 0.0001 | 0.001 | 0.3 | |
| 4th min (symbols n) | 125 [112; 130] | 101 [87; 116] | 101 [65; 113] | 0.0006 | <0.0001 | 0.5 | |
Q1, 25th percentile; Q3, 75th percentile; RT, reaction time; MS, missed signals; Words, 10 words memorizing test; Numbers, 10 numbers memorizing test; Syllables, 10 nonsense syllable memorizing test; 1st min, processed symbols in the 1st min; 4th min, processed symbols in 4th min.
At the next stage we conducted the analysis of the relative changes in postoperative cognitive indicators (a percentage of relative changes as compared to baseline) in the groups with and without training. It was found that the patients with training showed a lower frequency of a 20% decrease in cognitive functions. According to the results of the Bourdon test, the workability index (the number of processed symbols during the 1st minute of the test) worsened in 14% of patients in the training group and in 24% of patients without training (P=0.04). Moreover, patients without training were more likely to have higher number of errors in the Bourdon test—in 24% and 39%, respectively (P=0.02). The assessing the psychomotor and executive functions (the test of functional mobility of nervous processes) allowed to reveal an increase of the missed signals in 21% of patients with cognitive training and in 30% without training (P=0.049). The data of test “Performance of the brain responses to feedback” showed an increase of errors in 7% of patients with cognitive training, and, respectively, in 15% without training (P=0.04). Deterioration of short-term memory (≥20% decrease in the number of memorized syllables) was detected in 14% of patients undergoing cognitive training and in 27% cases in group without training (P=0.03).
EEG indicators
After surgery, ANOVA was performed with a between-subjects factor of Group (2 levels: cognitive/standard rehabilitation) and within-subjects factors of Examination Time (2 levels: before/after surgery), Area (5 levels: frontal, central, parietal, occipital and temporal) and Laterality (2 levels: left/right hemisphere) for the indicators of theta-1 rhythm power. The significance of the interaction Group × Examination Time [F(1, 60)=15.08, P=0.0003] was established. The results showed that patients undergoing cognitive training exhibit no signs of the negative dynamics of brain functioning compared with the group without training, which had an increase in the theta-1 power after surgery compared to the baseline [F(1, 60)=43.05, P≤0.0001] (Figure 2A,2B).
Discussion
The present study revealed impaired memory, attention, psychomotor and executive functions in cardiac surgery patients compared with healthy people of the same age on preoperative examination. Moreover, MRI revealed the signs of cerebral microangiopathy and cortical atrophy, which indicates that these patients suffered from chronic cerebral blood flow deterioration and neurodegeneration even before surgery. Thus, the selected cohort of cardiac surgery patients is vulnerable to the development of postoperative cognitive decline and aggravation of existing cognitive disorders.
We showed also that the multi-domains cognitive-motor training during the early postoperative period of CABG reduced the frequency of a decrease in cognitive functions. Positive changes have been achieved in the indicators of executive functions, attention and short-term memory. We assumed that a multitask approach to cognitive training can be promoting the involvement of various brain regions in the recovery process and contribute to improving their function and slow down the development of cognitive decline. Previously it was reported that cognitive training with two competing tasks is preferable to training with one task (36). The authors supposed that the use of cognitive-motor tasks for training contributes to better coordination of mental processes, which is necessary during the everyday activity and can affect quality of life (36-38). Moreover, it has been established that the neurocognitive functioning in the elderly in the cases of successful solution of cognitive tasks, and in the performance of competing tasks regardless of age requires of the enlargement of involvement of brain resources (38). Therefore, our results may reflect a transfer effect in the cognitive training group: when a training of any type of cognitive abilities improves the performance of other types (39).
In addition, the multi-domains cognitive-motor training resulted in a lower increase in slow-wave theta activity in the early postoperative period of CABG. As previously shown, a theta activity increase in postoperative period of cardiac surgery was associated with perioperative brain ischemia (40). It was also found that the progression of cognitive disorders was accompanied by the theta power increase (10,41). Compared with other neuroimaging methods, EEG is the most suitable and inexpensive method for studying of the brain activity in cardiac surgery patients. Since EEG is a non-invasive method with high temporal and spatial resolution, it is possible to evaluate the clinical markers of cognitive impairment, perioperative brain dynamics (42). The analysis of neuronal oscillations depending on their location, morphology and amplitude relative to the age and clinical status of the patient can also be used to objectively assess the effectiveness of cognitive training (43).
It should be noted that in the present study, the cognitive training course lasted a brief period in the postoperative period. The obtained results are important for cardiac patients as the way to form an adherence to treatment, to optimize the rehabilitation program in the subsequent postoperative period. Presumably, an increase in the duration of cognitive training will provide more pronounced positive effects. These are important questions for future studies.
Limitation
This study had a small set of patients. The cognitive training course lasted a brief period in the postoperative period (5–7 days after cardiac surgery). An identification the patients with better rehabilitation potential and a controlled randomized study to clarify the impact of the multitask approach to cognitive training on postoperative cognitive deficit would be useful in future studies.
Conclusions
The use of the multi-domains cognitive-motor training in the early postoperative period of CABG can be contributed to reduce the severity of postoperative cognitive decline due to the transfer effect, and presumably activated the processes of functional neuroplasticity, causing rearrangements of theta activity. To study of the effectivity of this type of rehabilitation and to properly implement it into clinical practice, more studies are needed.
Acknowledgments
Funding: The authors declare that this study received funding from the Federal State Ministry of Science and Education of Russian Federation [The Exploratory Research Study “Managing the risks associated with comorbidity in patients with circulatory system diseases through the application of innovative diagnostic and rehabilitation medical technologies” (No. 0419-2023-0001; protocol No. 10 dated 10/12/2020)]. The funder was not involved in the study design, collection, analysis, interpretation of data, the writing of this article or the decision to submit it for publication.
Footnote
Reporting Checklist: The authors have completed the CONSORT reporting checklist. Available at https://jxym.amegroups.com/article/view/10.21037/jxym-22-37/rc
Trial Protocol: Available at https://jxym.amegroups.com/article/view/10.21037/jxym-22-37/tp
Data Sharing Statement: Available at https://jxym.amegroups.com/article/view/10.21037/jxym-22-37/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jxym.amegroups.com/article/view/10.21037/jxym-22-37/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and the study involving human participants was reviewed and approved by the Ethics Committee of Ethics Committee of Federal State Budgetary Institution of Research Institute of Complex Issues of Cardiovascular Diseases (protocol No. 10 dated 10/12/2020). The patients provided their written informed consent to participate in this study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Cropsey C, Kennedy J, Han J, et al. Cognitive Dysfunction, Delirium, and Stroke in Cardiac Surgery Patients. Semin Cardiothorac Vasc Anesth 2015;19:309-17. [Crossref] [PubMed]
- Tarasova IV, Trubnikova OA, Syrova ID, et al. Long-Term Neurophysiological Outcomes in Patients Undergoing Coronary Artery Bypass Grafting. Braz J Cardiovasc Surg 2021;36:629-38. [Crossref] [PubMed]
- Bhushan S, Li Y, Huang X, et al. Progress of research in postoperative cognitive dysfunction in cardiac surgery patients: A review article. Int J Surg 2021;95:106163. [Crossref] [PubMed]
- Belrose JC, Noppens RR. Anesthesiology and cognitive impairment: a narrative review of current clinical literature. BMC Anesthesiol 2019;19:241. [Crossref] [PubMed]
- Patel N, Minhas JS, Chung EM. Risk Factors Associated with Cognitive Decline after Cardiac Surgery: A Systematic Review. Cardiovasc Psychiatry Neurol 2015;2015:370612. [Crossref] [PubMed]
- Benwell CSY, Davila-Pérez P, Fried PJ, et al. EEG spectral power abnormalities and their relationship with cognitive dysfunction in patients with Alzheimer's disease and type 2 diabetes. Neurobiol Aging 2020;85:83-95. [Crossref] [PubMed]
- Bonnefond M, Kastner S, Jensen O. Communication between Brain Areas Based on Nested Oscillations. eNeuro 2017;4:ENEURO.0153-16.2017.
- Başar E. The theory of the whole-brain-work. Int J Psychophysiol 2006;60:133-8. [Crossref] [PubMed]
- Shibata T, Musha T, Kosugi Y, et al. Altered Neuronal Activity Topography Markers in the Elderly with Increased Atherosclerosis. Front Aging Neurosci 2017;9:216. [Crossref] [PubMed]
- Babiloni C, Blinowska K, Bonanni L, et al. What electrophysiology tells us about Alzheimer's disease: a window into the synchronization and connectivity of brain neurons. Neurobiol Aging 2020;85:58-73. [Crossref] [PubMed]
- Tarasova IV, Trubnikova OA, Barbarash OL. EEG and Clinical Factors Associated with Mild Cognitive Impairment in Coronary Artery Disease Patients. Dement Geriatr Cogn Disord 2018;46:275-84. [Crossref] [PubMed]
- Ludyga S, Gerber M, Pühse U, et al. Systematic review and meta-analysis investigating moderators of long-term effects of exercise on cognition in healthy individuals. Nat Hum Behav 2020;4:603-12. [Crossref] [PubMed]
- Schwarck S, Busse N, Ziegler G, et al. Heart Rate Variability During Physical Exercise Is Associated With Improved Cognitive Performance in Alzheimer's Dementia Patients-A Longitudinal Feasibility Study. Front Sports Act Living 2021;3:684089. [Crossref] [PubMed]
- van de Ven RM, Murre JM, Veltman DJ, et al. Computer-Based Cognitive Training for Executive Functions after Stroke: A Systematic Review. Front Hum Neurosci 2016;10:150. [Crossref] [PubMed]
- Ballesteros S, Voelcker-Rehage C, Bherer L. Editorial: Cognitive and Brain Plasticity Induced by Physical Exercise, Cognitive Training, Video Games, and Combined Interventions. Front Hum Neurosci 2018;12:169. [Crossref] [PubMed]
- Ajtahed SS, Rezapour T, Etemadi S, et al. Efficacy of Neurocognitive Rehabilitation After Coronary Artery Bypass Graft Surgery in Improving Quality of Life: An Interventional Trial. Front Psychol 2019;10:1759. [Crossref] [PubMed]
- Greaves D, Psaltis PJ, Lampit A, et al. Computerised cognitive training to improve cognition including delirium following coronary artery bypass grafting surgery: protocol for a blinded randomised controlled trial. BMJ Open 2020;10:e034551. [Crossref] [PubMed]
- Nouchi R, Taki Y, Takeuchi H, et al. Reading Aloud and Solving Simple Arithmetic Calculation Intervention (Learning Therapy) Improves Inhibition, Verbal Episodic Memory, Focus Attention and Processing Speed in Healthy Elderly People: Evidence from a Randomized Controlled Trial. Front Hum Neurosci 2016;10:217. [Crossref] [PubMed]
- Tait JL, Duckham RL, Milte CM, et al. Influence of Sequential vs. Simultaneous Dual-Task Exercise Training on Cognitive Function in Older Adults. Front Aging Neurosci 2017;9:368. [Crossref] [PubMed]
- Ghai S, Ghai I, Effenberg AO. Effects of dual tasks and dual-task training on postural stability: a systematic review and meta-analysis. Clin Interv Aging 2017;12:557-77. [Crossref] [PubMed]
- Talamonti D, Vincent T, Fraser S, et al. The Benefits of Physical Activity in Individuals with Cardiovascular Risk Factors: A Longitudinal Investigation Using fNIRS and Dual-Task Walking. J Clin Med 2021;10:579. [Crossref] [PubMed]
- Bech SR, Kjeldgaard-Man L, Sirbaugh MC, et al. Attentional Capacity during Dual-task Balance Performance Deteriorates with Age before the Sixties. Exp Aging Res 2022;48:86-98. [Crossref] [PubMed]
- Gallou-Guyot M, Mandigout S, Combourieu-Donnezan L, et al. Cognitive and physical impact of cognitive-motor dual-task training in cognitively impaired older adults: An overview. Neurophysiol Clin 2020;50:441-53. [Crossref] [PubMed]
- Akin H, Senel A, Taskiran H, et al. Do motor-cognitive and motor-motor dual task training effect differently balance performance in older adults? Eur Geriatr Med 2021;12:371-8. [Crossref] [PubMed]
- Cisneros E, Beauséjour V, de Guise E, et al. The impact of multimodal cognitive rehabilitation on executive functions in older adults with traumatic brain injury. Ann Phys Rehabil Med 2021;64:101559. [Crossref] [PubMed]
- Sarasso E, Agosta F, Piramide N, et al. Brain activity of the emotional circuit in Parkinson's disease patients with freezing of gait. Neuroimage Clin 2021;30:102649. [Crossref] [PubMed]
- Johansson H, Ekman U, Rennie L, et al. Dual-Task Effects During a Motor-Cognitive Task in Parkinson's Disease: Patterns of Prioritization and the Influence of Cognitive Status. Neurorehabil Neural Repair 2021;35:356-66. [Crossref] [PubMed]
- Syrova ID, Lozhkin IS, Trubnikova OA, et al. Cerebrovascular complications of patients with coronary heart disease who underwent coronary bypass surgery (five-year follow-up). Creative Cardiology 2020;14:313-23.
- Tarasova IV, Razumnikova OA, Trubnikova OA, et al. Neurophysiological correlates of postoperative cognitive disorders. Zh Nevrol Psikhiatr Im S S Korsakova 2021;121:18-23. [Crossref] [PubMed]
- Nasreddine ZS, Phillips NA, Bédirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc 2005;53:695-9. [Crossref] [PubMed]
- Beck AT, Guth D, Steer RA, et al. Screening for major depression disorders in medical inpatients with the Beck Depression Inventory for Primary Care. Behav Res Ther 1997;35:785-91. [Crossref] [PubMed]
- Rose GA, Blackburn H. Cardiovascular survey methods. Monogr Ser World Health Organ 1968;56:1-188. [PubMed]
- Voytenko VP. Zdorove zdorovyih [Healthy health]. Kyiv: Zdorov’ya, 1991:248.
- Trubnikova OA, Tarasova IV, Moskin EG, et al. Beneficial Effects of a Short Course of Physical Prehabilitation on Neurophysiological Functioning and Neurovascular Biomarkers in Patients Undergoing Coronary Artery Bypass Grafting. Front Aging Neurosci 2021;13:699259. [Crossref] [PubMed]
- Gnedovskaya EV, Dobrynina LA, Krotenkova MV, et al. MRI in the assessment of cerebral small vessel disease. Annals of Clinical and Experimental Neurology 2018;12:61-8.
- Petrigna L, Thomas E, Gentile A, et al. Assessment of dual task conditions for static posture control in the elderly: a systematic review and meta-analysis protocol. Syst Rev 2019;8:188. [Crossref] [PubMed]
- Zhavoronkova LA, Maksakova OA, Shevtsova TP, et al. Dual-tasks is an indicator of cognitive deficit specificity in patients after traumatic brain injury. Zh Nevrol Psikhiatr Im S S Korsakova 2019;119:46-52. [Crossref] [PubMed]
- Dupuy EG, Besnier F, Gagnon C, et al. COVEPIC (Cognitive and spOrt Virtual EPIC training) investigating the effects of home-based physical exercise and cognitive training on cognitive and physical functions in community-dwelling older adults: study protocol of a randomized single-blinded clinical trial. Trials 2021;22:505. [Crossref] [PubMed]
- Strobach T. The dual-task practice advantage: Empirical evidence and cognitive mechanisms. Psychon Bull Rev 2020;27:3-14. [Crossref] [PubMed]
- Tarasova IV, Akbirov RM, Tarasov RS, et al. Electric brain activity in patients with simultaneous coronary artery bypass grafting and carotid endarterectomy. Zh Nevrol Psikhiatr Im S S Korsakova 2019;119:41-7. [Crossref] [PubMed]
- Babiloni C, Arakaki X, Azami H, et al. Measures of resting state EEG rhythms for clinical trials in Alzheimer's disease: Recommendations of an expert panel. Alzheimers Dement 2021;17:1528-53. [Crossref] [PubMed]
- Milne B, Gilbey T, Gautel L, et al. Neuromonitoring and Neurocognitive Outcomes in Cardiac Surgery: A Narrative Review. J Cardiothorac Vasc Anesth 2022;36:2098-113. [Crossref] [PubMed]
- McDevitt WM, Gul T, Jones TJ, et al. Perioperative electroencephalography in cardiac surgery with hypothermic circulatory arrest: a narrative review. Interact Cardiovasc Thorac Surg 2022;35:ivac198. [Crossref] [PubMed]
Cite this article as: Syrova ID, Tarasova IV, Trubnikova OA, Kupriyanova DS, Sosnina AS, Temnikova TB, Barbarash OL. A multitask approach to prevention of the cognitive decline after coronary artery bypass grafting: a prospective randomized controlled study. J Xiangya Med 2023;8:2.


