
Comparison of implantation depth between three-cusp and cusp-overlap views for a self-expanding transcatheter heart valve: a cross-sectional analysis
Introduction
Precise positioning is an important prerequisite of procedural success for transcatheter aortic valve replacement (TAVR) (1,2). Malpositioning increases the risk of device embolization, device failure in terms of paravalvular regurgitation (PVR) or increased transvalvular gradients, and occurrence of conduction disturbances and permanent pacemaker implantation (PPI), particularly in the event of low prosthesis positioning (2,3). In recent years, novel techniques have emerged to optimize prosthesis deployment. The cusp-overlap view (COV) technique is being increasingly used for precise implantation of the Evolut platform, with higher than usual positioning and consequently lower PPI rates (4-6). In the COV, the nadir of the left and right coronary cusp (LCC, RCC) overlap, and the left ventricular outflow tract (LVOT) can be appreciated without foreshortening, whereas in the traditional 3-cusp view (3-CV), the prosthesis may appear higher than it actually is.
The standard implantation view for the self-expanding ACURATE neo2 (Neo2) transcatheter heart valve (THV) is the 3-CV (7). Thus far, except for a case report (8) no systematic data exists regarding the implementation of the COV technique for the implantation of the ACURATE platform. We assumed that in the COV the implantation depth (ID) might be greater than in the 3-CV with the clinical implication of higher PPI rates.
The aim of the present study was to determine the ID measured at the non-coronary cusp (NCC) in the COV and compare it with the ID measured in the traditional 3-CV among Neo2 recipients. We present the following article in accordance with the STROBE reporting checklist (available at https://jxym.amegroups.com/article/view/10.21037/jxym-22-26/rc).
Methods
Study design
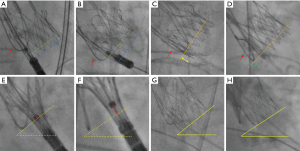
In this retrospective cross-sectional analysis, consecutive patients who underwent transfemoral TAVR for native aortic stenosis using the Neo2 THV in our center between June 2021 and March 2022 were considered. Inclusion criteria were the availability of final aortography and/or fluoroscopy in both the 3-CV and the COV view and the placement of a 0.035-inch wire in the NCC as landmark for the annular plane throughout the entire procedure. The latter allows for clear visualization of the nadir and hence precise measurement of the ID (Figure 1) (9). Exclusion criteria were TAVR for pure aortic regurgitation, bicuspid aortic valve, degenerated surgical or transcatheter bioprosthesis, and the use of alternative access routes. Further excluded were patients without aortography and/or fluoroscopy available in both the 3-CV and COV, those in whom the wire technique was not applied, those with cases of severe parallax as defined further below, and those in whom both the 3-CV and COV were available, but one of the angulations did not match the multidetector computed tomography (MDCT)-derived implantation view with a deviation exceeding >3° in either the 3-CV or the COV (referred to as incongruent).
Indications for TAVR, including the access route and type and size of the prosthesis, were discussed within a heart team consisting of a cardiac surgeon, an interventional cardiologist, and a cardiac anesthetist in adherence to current guidelines (10). All procedures were performed in a hybrid operating room with conscious sedation. Baseline characteristics including demographics, co-morbidities, risk scores, and echocardiography data as well as procedural data and in-hospital outcomes were prospectively documented in a dedicated database. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the ethics committee of the University of Giessen and individual consent for this retrospective analysis was waived.
ID measurements
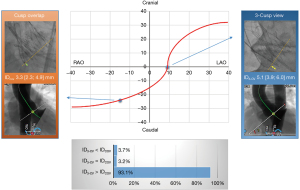
The MDCT-derived 3-CV was verified angiographically and adjusted if necessary. After adjustment of the 3-CV, the COV was corrected accordingly. After successful deployment of the prosthesis, cine-fluoroscopic images were obtained in both the 3-CV and COV. However, the final c-arm position was at the discretion of the operator and was adjusted to reduce radiation dose or improve image degradation in very extreme angulations. The difference between the 3-CV and the COV [∆ (3-CV–COV)] was determined in the anterior-oblique and cranio-caudal angulation. Severe parallax was defined as non-perpendicular prosthesis alignment with an incongruence of the lower crown margin exceeding the opposite lower cell row, whereas mild parallax was defined as incongruence of the lower crown margin exceeding only half of the opposite lower cell row (Figure 1A,1B). All cine-fluoroscopy images were analyzed off-line on a dedicated workstation (Syngo, Siemens, Forchheim, Germany) using Table-to-Object-Distance automatic calibration. The ID was defined as the distance between the inflow part of the prosthesis (lower crown) and the aortic annular plane indicated by the positioning wire in the NCC and was measured in the 3-CV (ID3-CV) and in the COV (IDCOV) (Figure 1C,1D).
Pre-deployment assessment of prosthesis position
In a subset of patients, fluoroscopic angulations were available both in the 3-CV and the COV after completion of step 1 of the prosthesis implantation (release of the upper crown and stabilization arches). Step 1 was monitored in the 3-CV, and after completion of step 1 the position was recorded using cine-fluoroscopy in the 3-CV and immediately thereafter in the COV. Measurements were made between the central part of the radiopaque positioning marker and the annular plane. For the latter, the angle between a virtual horizontal line and the inflow part of the deployed prosthesis was taken into account (Figure 1E-1H).
Computed tomography measurements
Pre-procedural MDCT of the entire aorta and ilio-femoral arteries was performed as part of the routine clinical workup using a 64-slice or a 192-slice dual-source scanner (Somatom Definition or Force, Siemens Healthcare, Forchheim, Germany), as previously described (11).
MDCT datasets were analyzed in a standard fashion using dedicated software (3mensio, Piemedical, The Netherlands). The 3-CV and the COV were determined as described earlier (4).
Outcomes of interest
The main outcome of interest was the difference between ID3-CV and IDCOV at the NCC [∆(ID3-CV − IDCOV)] and the distribution of cases in which ID3-CV was larger, similar, or smaller than IDCOV. Secondary outcome measures were in-hospital outcomes according to the Valve Academic Research Consortium (VARC)-3 criteria and 30-day all-cause mortality (12).
Statistical analysis
Continuous variables are given as median and interquartile range (IQR); categorical data are presented as numbers and percentages. Continuous data were compared using the Wilcoxon rank-sum test and the Wilcoxon signed-rank test for paired analyses; categorical data were compared using the Fisher’s exact test or Chi-squared test, as appropriate. The correlation of continuous variables was determined using the Pearson method. Agreement between the various measurement methods of the ID was examined with the Bland-Altman test. Linear regression analysis was used to determine independent predictors of ∆(ID3-CV − IDCOV) by entering all covariates into the multivariable analysis that in the univariate analysis had P values ≤0.1. A two-sided P value <0.05 was considered significant. All analyses were performed using STATA IC version 16.1 (StataCorp LCC, College Station, Texas, USA) and R version 4.1.3 (R Core Team (2022). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL https://www.R-project.org/).
Results
Study cohort
A total of 290 patients underwent transfemoral TAVR using the Neo2 device in our institution between June 2021 and March 2022. After exclusion of 101 patients for various reasons, the final study cohort consisted of 189 patients (Figure 2). The baseline characteristics of the final study population are shown in Table 1. The median age was 82 years (IQR, 78; 85 years) and 54.0% were female. Procedural data and in-hospital outcomes are provided in Table 2.
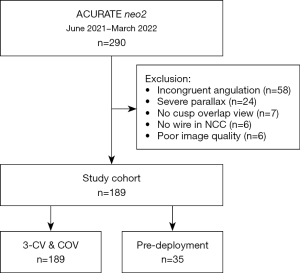
Table 1
| Variables | Total cohort (n=189) |
|---|---|
| Age, years | 82 [78; 85] |
| Female sex | 102 (54.0) |
| Body mass index, kg/m2 | 27.3 [24.3; 31.6] |
| EuroSCORE II, % | 2.6 [1.7; 4.5] |
| eGFR, mL/min/1.73 m2 | 69 [48; 88] |
| Hypertension | 164 (86.8) |
| Diabetes | 65 (34.4) |
| Hyperlipidemia | 108 (57.1) |
| Coronary artery disease | 104 (55.0) |
| Atrial fibrillation | 80 (42.3) |
| Prior permanent pacemaker | 26 (13.8) |
| Ejection fraction, % | 65 [60; 65] |
| Mean gradient, mmHg | 42 [31; 50] |
| Aortic valve area, cm2 | 0.8 [0.6; 0.9] |
| AVCS, AU | 2,292 [1,486; 3,111] |
| Aorto-annular angle, ° | 49 [43; 55] |
Values denote median [interquartile range] or n (%). AVCS, aortic valve calcium score; AU, Agatston units; eGFR, estimated glomerular filtration rate.
Table 2
| Variables | Total cohort (n=189) |
|---|---|
| Prosthesis size | |
| S, 23 mm | 42 (22.2) |
| M, 25 mm | 75 (39.7) |
| L, 27 mm | 72 (38.1) |
| Procedural duration, min | 39 [34; 48] |
| Fluoroscopy time, min | 8.1 [6.1; 11.0] |
| Contrast agent, mL | 25 [20; 43] |
| Pre-dilatation | 168 (88.9) |
| Post-dilatation | 67 (35.4) |
| Malpositioning | 3 (1.6) |
| Ejection fraction post, % | 65 [65; 65] |
| Mean gradient post, mmHg | 9 [7; 12] |
| Aortic valve area post, cm2 | 1.7 [1.5; 2.0] |
| Paravalvular regurgitation ≥ moderate | 1 (0.5) |
| Technical success (VARC-3) | 180 (95.2) |
| Device success (VARC-3) | 130 (68.8) |
| Complications | |
| 30-day all-cause mortality | 1 (0.6) |
| Early safety endpoint (VARC-3) | 31 (16.4) |
| Aortic dissection | 0 |
| Annular rupture | 0 |
| Coronary obstruction | 0 |
| Conversion to sternotomy | 0 |
| Multiple valve implantation | 1 (0.5) |
| Device embolization | 0 |
| Major vascular complication | 10 (5.3) |
| Severe bleeding (types 2–4) | 11 (5.8) |
| Any stroke | 3 (1.6) |
| Acute kidney injury (stages 2–4) | 2 (1.1) |
| Pacemaker implantation | 12 (6.3) |
| New-onset LBBB | 23 (12.2) |
Values denote median [interquartile range] or n (%). S, small; M, medium; L, large; LBBB, left bundle branch block; VARC, Valve Academic Research Consortium.
Implantation views and depth measurements
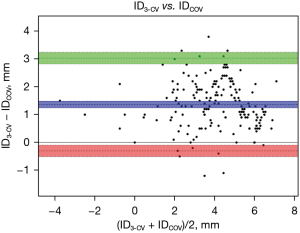
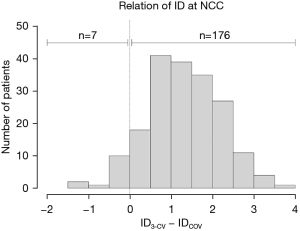
The results of the ID measurements are summarized in Table 3 and in the Figure 3. In the anterior-oblique angulation, ∆(3-CV–COV) was 24° (IQR, 21°; 27°), and in the cranio-caudal angulation, the difference was 25° (IQR, 21°; 28°). The median ID3-CV was 5.1 mm (IQR, 3.9; 6.0 mm), and the median IDCOV was 3.3 mm (IQR, 2.3; 4.9 mm), with a high correlation between the two values (R=0.89; P<0.001) and an excellent agreement in the Bland-Altman analysis (Figure 4). ∆(ID3-CV − IDCOV) was 1.3 mm (IQR, 0.9; 1.9 mm), and ID3-CV was usually greater than IDCOV (n=176; 93.1%) (Figure 5).
Table 3
| Variables | Total cohort (n=189) |
|---|---|
| ID3-CV NCC, mm | 5.1 [3.9; 6.0] |
| IDCOV NCC, mm | 3.3 [2.3; 4.9] |
| ∆(ID3-CV − IDCOV), mm | 1.3 [0.9; 1.9] |
| Relation of ID at NCC | |
| ID3-CV > IDCOV | 176 (93.1) |
| ID3-CV = IDCOV | 6 (3.2) |
| ID3-CV < IDCOV | 7 (3.7) |
| ∆(ID3-CV − IDCOV) ≥1 mm | 132 (69.8) |
| Angulation 3-CV, ° | |
| Anterior-oblique | 9 [2; 15] (LAO) |
| Cranio-caudal | 0 [CAU 5; CRA 7] |
| Angulation COV, ° | |
| Anterior-oblique | 15 [8; 21] (RAO) |
| Cranio-caudal | 24 [18; 29] (CAU) |
| ∆(3-CV − COV), ° | |
| Anterior-oblique | 24 [21; 27] |
| Cranio-caudal | 25 [21; 28] |
Values denote median [Interquartile range] and n (%). ∆(ID3-CV − IDCOV), difference between implantation depth in the 3-CV and the COV; ∆(3-CV − COV), difference between 3-CV and COV; ID3-CV, implantation depth measured in the 3-cusp view; IDCOV, implantation depth measured in the cusp-overlap view; 3-CV, 3-cusp view; COV, cusp-overlap view; NCC, non-coronary cusp; RAO, right anterior oblique; LAO, left anterior oblique; CAU, caudal; CRA, cranial.
Positioning prior to valve deployment
In the subset of patients (n=35) with available measurements of the distance between the radiopaque marker band and the annular plane prior to step 2 (full prosthesis deployment), the distance in the 3-CV was smaller than in the COV [1.0 (IQR, 0; 1.4) vs. 2.3 (IQR, 1.3; 2.8) mm; P<0.001].
Predictors of ∆(ID3-CV − IDCOV)
In the multivariable analysis, only a larger ∆(3-CV – COV) in the cranio-caudal angulation was independently related to ∆(ID3-CV − IDCOV) (Table 4).
Table 4
| Variables | Coefficient (95% CI) | P | Adjusted coefficient (95% CI) | P |
|---|---|---|---|---|
| ∆(3-CV − COV) anterior-oblique, per ° | −0.023 (−0.046; 0.001) | 0.051 | – | – |
| ∆(3-CV − COV) cranio-caudal, per ° | 0.043 (0.020; 0.067) | <0.001 | 0.054 (0.025; 0.084) | <0.001 |
| Aorto-annular angle, per ° | 0.012 (−0.002; 0.026) | 0.085 | – | – |
| Mild parallax | 0.038 (−0.236; 0.313) | 0.783 | – | – |
∆(ID3-CV − IDCOV), difference between implantation depth in the 3-CV and the COV; ∆(3-CV − COV), difference between 3-CV and COV; ID3-CV, implantation depth measured in the 3-cusp view; IDCOV, implantation depth measured in the cusp-overlap view; 3-CV, 3-cusp view; COV, cusp-overlap view; CI, confidence interval.
Discussion
The present study represents the first comparison of the ID measurement in the 3-CV versus the COV. The main finding is that ID3-CV and IDCOV usually differ, with predominantly greater values for ID3-CV than for IDCOV. Accordingly, pre-implantation assessment of the device position revealed that the prosthesis appears to be higher (more aortic) in the COV than in the 3-CV.
Technical considerations
Our findings comparing the 3-CV and the COV in terms of ID and pre-implantation position are counter-intuitive and difficult to explain. Theoretically, the COV represents the most appropriate plane for assessment of the ID at the NCC, as it allows for a non-foreshortened view of the LVOT (4). It has been suggested that implantation using the COV enables precise positioning and is associated with lower rates of PPI (5,6,13). Therefore, one would expect that the IDCOV would appear greater than the ID3-CV, whereas the pre-implantation position should appear lower in the COV than in the 3-CV.
In a recent publication, of 444 TAVR cases using the Evolut R prosthesis, 161 pairs were identified by means of propensity score matching to compare outcomes of implantation in the COV versus the standard implantation view that was not further specified. Among COV cases, the ID at the NCC was smaller (4.2 vs. 5.3 mm; P<0.001), and the PPI rate was lower (11.8% vs. 21.7%; P=0.03) (6). In a study by Sammour et al., among recipients of the SAPIEN 3 balloon-expandable device, a high implantation technique performed in the COV led to a smaller ID (1.5±1.6 vs. 3.2±1.9 mm; P<0.001) along with lower PPI rates (5.5% vs. 13.1%; P<0.001) in comparison with procedures using the 3-CV (13). Even though the ID in the COV groups was smaller than that in the standard implantation groups, it is important to note that in those studies comparisons were made between COV and 3-CV groups, and therefore the difference may rather have been a result of a higher implantation strategy in the COV group. This is in contrast to the present study in which IDCOV was compared with ID3-CV within the same patient, thus representing the intra-individual difference between the two implantation views. Nonetheless, we assume that the discrepancy between ID3-CV and IDCOV found in the present analysis, with values of ID3-CV being mostly greater, would be found for any valve type, provided intra-individual measurements are available.
Clinical implications
Data comparing implantation in the 3-CV vs. the COV are limited to retrospective observational studies with small to moderate sample sizes and are essentially confined to the self-expanding Evolut platform and the balloon-expandable SAPIEN 3 device. However, all studies consistently demonstrated that implantation using the COV technique markedly reduces PPI rates when compared with the standard implantation technique in the 3-CV. For the Evolut platform, reduced PPI rates were reported to range from 6.5% to 13.1% in the COV group vs. 17.8% to 30.9% in the 3-CV group (5,6).
The most urgent question is whether the application of the COV for the implantation of the ACURATE neo2 valve will likewise result in lower PPI rates. The present study cannot ultimately address this specific question, as the final release of the prosthesis was exclusively performed in the 3-CV. Nonetheless, post-deployment assessments of the ID in the 3-CV and the COV were available in the entire study cohort, and in a subset of patients, pre-implantation views were obtained both in the 3-CV and COV immediately before step 2.
Based on the current standard positioning technique in the 3-CV, which takes into account that the radiopaque positioning marker on the delivery catheter should be at the annular level, our results suggest that the implantation of the Neo2 in the COV would result in a lower position as the device appears to be higher in the COV than in the 3-CV. This in turn may serve to increase the PPI rate rather than to reduce it. Furthermore, it may be assumed that the standard technique for implantation of the Neo2 in the 3-CV overestimates the “true” ID (i.e., the prosthesis position appears to be lower than it actually is). Therefore, application of the COV for implantation of the Neo2 will require a revision of the positioning strategy that should involve establishing a higher position of the radiopaque marker that is slightly above the annular plane.
Further differences between device platforms that should be considered involve the mechanism of deployment as well as the procedural phase and the marker used for the assessment of the device position. The release of the Evolut valve is from the bottom up, and the COV is predominantly used for the assessment of the initial device position (namely, the inflow part) and during the early phase of deployment. In contrast, the Neo2 device is characterized by a top-down deployment, and the positioning relies on the radiopaque positioning marker prior to step 2. Finally, in addition to improved assessment of the device position, use of the COV also involves less interaction of the Evolut prosthesis in the LVOT, which does not apply to the Neo2 system.
Determinants of the difference in ID ∆(ID3-CV − IDCOV)
Our predictor analysis revealed that only the difference in the cranio-caudal angulation is independently related to ∆(ID3-CV – IDCOV). Therefore, especially in the event of larger differences in the cranio-caudal angulation, ∆(ID3-CV – IDCOV) should be expected to be larger and must be taken into account for a complementary assessment in both angulations.
Limitations
The results of the present study have to be interpreted in the light of several limitations. IDs were measured retrospectively, even though all other data were collected prospectively, and measurements were not adjudicated by a core laboratory. Furthermore, the ID was determined only at the NCC, although this has been shown to be mostly indicative for the prediction of conduction disturbances (6,14). The analysis is purely observational without any intervention or randomization; hence our findings are at best hypothesis generating and must be confirmed by adequately designed studies. Due to the exclusion of a relatively high number of cases, reporting of clinical outcomes may be biased; however, the focus of the present analysis was on the ID and not on clinical outcomes.
Conclusions
ID measurement of the Neo2 valve revealed predominantly greater values in the 3-CV than in the COV. Accordingly, pre-implantation assessment of the prosthesis showed that the prosthesis appeared to be in a higher position in the COV than in the 3-CV. Hence, based on the current positioning strategy we assume that implantation of the Neo2 in the COV would result in lower positioning than in the 3-CV.
Acknowledgments
We thank Elizabeth Martinson, PhD, from the KHFI Editorial Office for her editorial assistance.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jxym.amegroups.com/article/view/10.21037/jxym-22-26/rc
Data Sharing Statement: Available at https://jxym.amegroups.com/article/view/10.21037/jxym-22-26/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jxym.amegroups.com/article/view/10.21037/jxym-22-26/coif). WKK received proctor/speaker honoraria from Abbott, Boston Scientific, Edwards Lifesciences, Medtronic, Meril Life Sciences. YHC received personal fees from Edwards Lifesciences, Cytosorbents, CryoLife, Getinge. CWH is advisory board member to Medtronic. EIC received personal fees from Boston Scientific. WKK serves as an unpaid editorial board member of Journal of Xiangya Medicine from November 2021 to October 2023. The other author has no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by ethics board of Justus-Liebig University of Giessen/Marburg, Germany and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Vavuranakis M, Kariori M, Scott L, et al. Impact of "high" implantation on functionality of self-expandable bioprosthesis during the short- and long-term outcome of patients who undergo transcatheter aortic valve implantation: Is high implantation beneficial? Cardiovasc Ther 2018;36:e12330. [Crossref] [PubMed]
- Piayda K, Hellhammer K, Veulemans V, et al. Navigating the "Optimal Implantation Depth" With a Self-Expandable TAVR Device in Daily Clinical Practice. JACC Cardiovasc Interv 2020;13:679-88. [Crossref] [PubMed]
- Kim WK, Schäfer U, Tchetche D, et al. Incidence and outcome of peri-procedural transcatheter heart valve embolization and migration: the TRAVEL registry (TranscatheteR HeArt Valve EmboLization and Migration). Eur Heart J 2019;40:3156-65. [Crossref] [PubMed]
- Tang GHL, Zaid S, Michev I, et al. "Cusp-Overlap" View Simplifies Fluoroscopy-Guided Implantation of Self-Expanding Valve in Transcatheter Aortic Valve Replacement. JACC Cardiovasc Interv 2018;11:1663-5. [Crossref] [PubMed]
- Mendiz OA, Noč M, Fava CM, et al. Impact of Cusp-Overlap View for TAVR with Self-Expandable Valves on 30-Day Conduction Disturbances. J Interv Cardiol 2021;2021:9991528. [Crossref] [PubMed]
- Pascual I, Hernández-Vaquero D, Alperi A, et al. Permanent Pacemaker Reduction Using Cusp-Overlapping Projection in TAVR: A Propensity Score Analysis. JACC Cardiovasc Interv 2022;15:150-61. [Crossref] [PubMed]
- Kim WK, Hengstenberg C, Hilker M, et al. Transcatheter aortic valve implantation with the ACURATE neo valve: indications, procedural aspects and clinical outcomes. EuroIntervention 2020;15:e1571-9. [Crossref] [PubMed]
- Wong I, Bajoras V, Wang X, et al. Technical Considerations for Transcatheter Aortic Valve Replacement With the Navitor Transcatheter Heart Valve. JACC Cardiovasc Interv 2021;14:e259-61. [Crossref] [PubMed]
- Kim WK, Doerr O, Renker M, et al. Initial experience with a novel, modular, minimalistic approach for transfemoral aortic valve implantation. Int J Cardiol 2021;332:54-9. [Crossref] [PubMed]
- Vahanian A, Beyersdorf F, Praz F, et al. 2021 ESC/EACTS Guidelines for the management of valvular heart disease. Eur Heart J 2022;43:561-632. [Crossref] [PubMed]
- Achenbach S, Delgado V, Hausleiter J, et al. SCCT expert consensus document on computed tomography imaging before transcatheter aortic valve implantation (TAVI)/transcatheter aortic valve replacement (TAVR). J Cardiovasc Comput Tomogr 2012;6:366-80. [Crossref] [PubMed]
- VARC-3 WRITING COMMITTEE. Valve Academic Research Consortium 3: Updated Endpoint Definitions for Aortic Valve Clinical Research. J Am Coll Cardiol 2021;77:2717-46. [Crossref] [PubMed]
- Sammour Y, Banerjee K, Kumar A, et al. Systematic Approach to High Implantation of SAPIEN-3 Valve Achieves a Lower Rate of Conduction Abnormalities Including Pacemaker Implantation. Circ Cardiovasc Interv 2021;14:e009407. [Crossref] [PubMed]
- Kim WK, Möllmann H, Walther T, et al. Predictors of permanent pacemaker implantation after ACURATE neo transcatheter heart valve implantation. Pacing Clin Electrophysiol 2021;44:410-5. [Crossref] [PubMed]
Cite this article as: Kim WK, Renker M, Choi YH, Hamm CW, Charitos EI. Comparison of implantation depth between three-cusp and cusp-overlap views for a self-expanding transcatheter heart valve: a cross-sectional analysis. J Xiangya Med 2022;7:28.


