
Laser capture microdissection in lung cancer: a narrative review
Introduction
Lung cancer is the most common cause of cancer mortality worldwide and its treatment is particularly challenging since most patients are diagnosed in advanced tumor stages without surgical resection option, with either metastatic disease or unresectable tumor (1).
The advent of increasingly sophisticated molecular characterization techniques, aimed at identifying predictive and prognostic biomarkers, and the progressive development of new targeted drugs have now widely paved the way to the new precision treatment era of non-small-cell lung cancer (NSCLC) (2).
A correct mutational analysis is dependent on the quantity and quality of nucleic acids retrieved from the pathological samples. Frequently, the only material available for molecular testing is a cytological specimen (effusion fluids, liquid-based preparations, conventional fine needle aspirations or cell blocks). In cytopathological samples, which account for around 40% of NSCLC biopsied cases, tumor cells are scattered and mixed with normal elements making selecting for tumor enrichment difficult (3). On the other hand, small lung biopsies often contain only few available tumor cells, as they are usually consisted for the most part of non-neoplastic cells such as fibroblasts and endothelial cells of the tumor stroma, adjacent normal tissue, inflammatory infiltrate, histiocytes, mesothelium and other cells among the more than 42 identifiable lung cell types (4). In addition, nucleic acids and proteins extracted from formalin-fixed paraffin-embedded (FFPE) specimens are often highly fragmented and/or chemically modified (5).
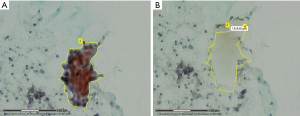
Since the 90s, the role played by laser capture microdissection (LCM) is precisely isolating tumor cells from the surrounding elements in order to increase the genomic and proteomic diagnostic test performances, especially enhancing sensitivity, and reliably overcoming the cellular heterogeneity, starting either from smear cytology samples, cell block specimens and fresh, frozen or FFPE tissues (Figure 1) (5-9).

Furthermore, it was found that in non-FFPE samples it is still feasible to maintain a good nucleic acid integrity for LCM up to 3 days if the tissue is kept at −80 ℃ (10). The procedure involves a laser excision (a laser cuts around the boundary of a selected area and successively a laser pulse forces the cells into a collecting device) and a high-resolution microscope usually coupled with a video system (Figures 2,3).

Due to the increasing necessity for lung cancer (and not only) molecular characterization in routine practice, there is as well an urgent need for an efficient total automatization of this procedure, in fact currently LCM has a daily-routine little use owed mostly to high costs and long cells selection and collection times, with rather a more extensive employment in the multi-omics research fields (13-15).
In this narrative review, we will provide a brief report about the feasible various applications of LCM in routine clinical practice lung cancer scenarios and we will take stock of the situation about the attempts to combine it with some newer diagnostic techniques. Finally, we summarize in Table S1 the main recent LCM progress in lung setting.
We present the following article in accordance with the Narrative Review reporting checklist (available at https://jxym.amegroups.com/article/view/10.21037/jxym-21-55/rc).
Methods
We performed a literature search (date of search: December 21, 2021) in PubMed (Medline) for studies published from January 1, 1995 to December 20, 2021 using a predefined search strategy combining the following search terms: “lung cancer” and “laser capture microdissection” requiring the term “laser” to appear in either the title or the abstract of the papers.
Articles satisfying the following inclusion criteria were included in our review (regardless of the study design): (I) study was written in English language; (II) the full article could be obtained.
Articles satisfying the following exclusion criteria were excluded in our review: (I) study was written in non-English language; (II) the full article was not available; (III) study was not related to lung cancer; (IV) study was not published in a peer-reviewed journal.
The literature review and the data extraction were conducted independently by two reviewers (D.S. and F.P.). A secondary search of the literature was manually conducted from the references of our primary search included papers by the application of the same inclusion and exclusion criteria. Doubts or disagreements regarding the inclusion or exclusion of manuscripts were resolved through a discussion between the reviewers until a consensus was reached (search strategy summary at Table 1 and detailed Medline search strategy at Table S2).
Table 1
| Items | Specifications |
|---|---|
| Date of search | 21-Dec-2021 |
| Databases and other sources searched | PubMed (Medline) |
| Search terms used | Search terms: “lung cancer” and “laser capture microdissection” |
| Timeframe | From January 1, 1995 to December 20, 2021 |
| Inclusion and exclusion criteria | Inclusion criteria: |
| (I) Study was written in English language; | |
| (II) The full article could be obtained | |
| Exclusion criteria: | |
| (I) Study was written in non-English language; | |
| (II) The full article was not available; | |
| (III) Study was not related to lung cancer; | |
| (IV) Study was not published in a peer-reviewed journal | |
| Selection process | The literature review and the data extraction were conducted independently by two reviewers (D.S. and F.P.) |
| A secondary search of the literature was manually conducted from the references of our primary search included papers by the application of the same inclusion and exclusion criteria | |
| Doubts or disagreements regarding the inclusion or exclusion of manuscripts were resolved through a discussion between the reviewers until a consensus was reached |
Discussion
Undissected samples with traditional tissue-block homogenization contain a tangled mixture of tumor and non-neoplastic cells. This heterogeneity and the usual low tumor content are the two major problems in the investigation of lung cancer molecular signatures in cell blocks because they determine the inability to perform an efficient neoplastic cells selection for molecular characterization, unlike what occurs with macrodissection carried out on histological sections from surgical resections. Hence cell blocks are typically used in their entirety by whole slide scrapes for DNA/RNA extraction, thus strongly diluting the tumor DNA/RNA content, obscuring signals from the malignant compartment and decreasing the sensitivity of the molecular assays by raising the limits of detection for genomic variants (16-18). To remedy this issue, it is possible to use a LCM system to precisely dissect the morphologically malignant cells and so enhance the desired cell population before subsequent nucleic acid or protein isolation. Manual microdissection (microscope plus sterile scalpel) is feasible with lower costs and greater temporal efficiency and throughput for tissue separation, although precision may not be as good as for LCM (19). Furthermore, both manual and laser techniques are subjected to a time-consuming and tedious user-dependent cell-by-cell selection of regions of interest (ROI) under direct microscopic visualization accomplished by a pathologist or cytotechnologist (20). In addition, laser-associated heat as well as the presence of nucleases or proteases tissue-specific (e.g., lung, pancreatic, spleen) may accelerate DNA, RNA and protein degradation processes, thereby a safety margin laser application and different protocols depending on the tissue type are employed (10,21).
A rapid and eventually user-independent ROI selection is achievable with the immunoguided LCM, based on cancer specific immunostaining (e.g., anti-cytokeratin-7 primary antibody for lung adenocarcinoma), even by the use of handheld and computer-aided laser devices (21-24).
Immunoguided LCM, compatible with both immuno-cyto/histochemistry or immunofluorescence targeting approaches, may be either human operator-based, computer-assisted via stain recognition algorithms or expression-based (16). In particular, this last user-independent method relies on a semiautomated identification and dissecting software in need of minimal supervision due to its ability to properly judge antibody staining. In immunoguided LCM the stained slices may also be previously covered with an ethylene-vinyl-acetate (EVA) membrane and then a laser irradiation can be performed on the whole slide: the heat derived from the localized energy absorption by the dark DAB (diaminobenzidine) stained tumor cells leads to the corresponding melting of the EVA membrane at the sites of most intensive staining. Subsequently, when the complete EVA membrane is removed, the attached tumor cells are isolated from the non-neoplastic elements, with an efficiency strongly related to immunostaining intensity and laser energy (19,23). An attempt to further optimize this process is the use of Vektor Black as chromogen, which provides to positive cells a dark black staining able of increasing the absorbed energy of the infrared laser irradiation better than slides stained with DAB (19). The immunoguided LCM has been even combined with a digital whole-slide scanning and image analysis performed before and after microdissection as a quality control protocol (19). However, it should be considered that the immunoguided LMC brings with it time, cost and technical issues related to the immunostaining steps, as well as their potential deleterious effects on the nucleic acid quality (25).
Conversely, a method that does not necessarily require immunostaining is the spatially invariant vector quantization, a pattern-matching algorithm for identification of specific cell types based on an iterative testing and real-time evaluation of match quality (16,24). With this kind of platform, the pathologist just has to identify the cell type or the morphologic pattern of interest and then the machine learning algorithm performs a whole-slide research to find similar features, including cell size, shape, nucleus and nucleolus (Figure 4).
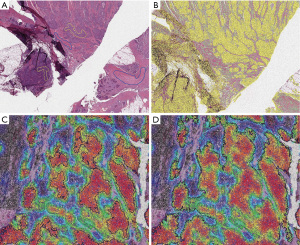
Selbach et al. in 2021 conceived a hyperspectral infrared microscopy LCM procedure, feasible on label-free or even on hematoxylin and eosin (H&E) stained tissue sections (13). This method is based on the Fourier transform infrared (FTIR) imaging technique, which assigns a vibrational spectrum to each tissue component at high spatial resolution (27). This fully automated approach relies on a trained random forest classifier able to correctly recognize each infrared pixel spectrum, distinguishing different types of tissue (e.g., normal, tumoral, inflamed) as well as various subtypes of thoracic tumors, and thus proceed to a 95% success rate ROI dissection (28).
A combined protocol of LCM and mass spectrometry (LCM-MS) on FFPE specimens of lung tissue, applicable even on the matrix-assisted laser desorption/ionization (MALDI) technique, has been recently proposed (29). The LCM-MS has the advantage of investigating the content of cells within their morphological context, even at single cell resolution (30). Nevertheless, it must be stated that larger sampling is needed in case of LCM for protein identification, since their analysis usually needs a larger amount of template compared to the few nanograms required by either PCR assays and next generation sequencing (NGS) technology, with which a wide range of molecular analysis (genomic, epigenomic and transcriptomic) can be performed with low quality/quantity material (31,32). In order to reduce sample loss and therefore improve sensitivity of LCM-based proteomics, different preparation protocols have been developed in the years (21,33-36).
Interestingly, LCM may also have a role in lung adenocarcinoma programmed death ligand-1 (PDL-1) expression assessment through Reverse Phase Protein Microarrays (RPPA), in fact this combination allows a continuous quantitative scaled detection with performances comparable to the immunohistochemistry (IHC), but potentially less dependent upon subjective operator evaluations or IHC clones employed, with an improved insight on immune cells classes and their spatial relationship with the tumour cells (37).
Finally, the opportunity to have LCM tools under the direct Pathology Department Laboratory Information System (LIS) control may allow the pathologist to speed up the procedure by immediately selecting the ROI, in the strength of his expertise and from what he has already investigated in the course of the diagnostic phase, thus being able to promptly provide a correct estimate of either the quantitative and qualitative adequacy of the specimen sent to the downstream molecular analyzes. Additionally, the progressive digitalisation of all the pathologist’s activities will lead to the development of a single fluent comprehensive diagnostic and molecular workflow, while the insertion of a proper LCM section in the pathology report would allow the full respect of the traceability criteria (synoptic report).
In conclusion, the LCM daily-routine application in clinical practice, after an initial great enthusiasm, is currently heavily constrained by numerous and well-known limitations (Table 2), nevertheless the development of tailor-made digital pathology tools and machine learning algorithms may lead to an efficacy and reliable, as well as rapid and sensitive, automatization of the LCM workflow and therefore result in a potential large-scale use, while the LCM combination with advanced high resolution techniques (e.g., MALDI) may open up new scenarios in the research setting (38-40).
Table 2
| Pros | Cons |
|---|---|
| Single cell precision | Expensive |
| Combination with single cell resolution techniques (e.g., MALDI) | Time-consuming |
| Semi/fully automated ROI selection (if computer-assisted LCM) | Laser-associated heat degradation |
| Single fluent diagnostic and molecular digitized workflow | Tedious user-dependent selection (especially if manual LCM) |
| Compliance with the traceability criteria (synoptic report) | Requires a pathologist or a cytotechnologist expertise (especially if manual LCM) |
| Nucleases and proteases tissue-specific presence | |
| Immunostaining issues (if immunoguided LCM) |
MALDI, matrix-assisted laser desorption/ionization; ROI, regions of interest; LCM, laser capture microdissection.
Conclusions
LCM is a reliable procedure for the investigation of protein and gene expression in specific areas of interest, especially crucial nowadays in the characterization of lung cancer molecular signatures. In the future, its reliability and ease of use will make LCM an essential step in the application of the numerous available downstream analyses.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Umberto Malapelle and Giancarlo Troncone) for the series “Predictive Molecular Pathology in Lung Cancer” published in Journal of Xiangya Medicine. The article has undergone external peer review.
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://jxym.amegroups.com/article/view/10.21037/jxym-21-55/rc
Peer Review File: Available at https://jxym.amegroups.com/article/view/10.21037/jxym-21-55/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jxym.amegroups.com/article/view/10.21037/jxym-21-55/coif). The series “Predictive Molecular Pathology in Lung Cancer” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- The International Agency for Research on Cancer (IARC). Global Cancer Observatory, Available online: http://globocan.iarc.fr/ (accessed 17 December 2021).
- Imyanitov EN, Iyevleva AG, Levchenko EV. Molecular testing and targeted therapy for non-small cell lung cancer: Current status and perspectives. Crit Rev Oncol Hematol 2021;157:103194. [Crossref] [PubMed]
- Yatabe Y, Dacic S, Borczuk AC, et al. Best Practices Recommendations for Diagnostic Immunohistochemistry in Lung Cancer. J Thorac Oncol 2019;14:377-407. [Crossref] [PubMed]
- Lin J, Marquardt G, Mullapudi N, et al. Lung cancer transcriptomes refined with laser capture microdissection. Am J Pathol 2014;184:2868-84. [Crossref] [PubMed]
- Didelot A, Kotsopoulos SK, Lupo A, et al. Multiplex picoliter-droplet digital PCR for quantitative assessment of DNA integrity in clinical samples. Clin Chem 2013;59:815-23. [Crossref] [PubMed]
- Maurer HC, Olive KP. Laser Capture Microdissection on Frozen Sections for Extraction of High-Quality Nucleic Acids. Methods Mol Biol 2019;1882:253-9. [Crossref] [PubMed]
- Emmert-Buck MR, Bonner RF, Smith PD, et al. Laser capture microdissection. Science 1996;274:998-1001. [Crossref] [PubMed]
- Chowdhuri SR, Xi L, Pham TH, et al. EGFR and KRAS mutation analysis in cytologic samples of lung adenocarcinoma enabled by laser capture microdissection. Mod Pathol 2012;25:548-55. [Crossref] [PubMed]
- Liu Y, Noon AP, Aguiar Cabeza E, et al. Next-generation RNA sequencing of archival formalin-fixed paraffin-embedded urothelial bladder cancer. Eur Urol 2014;66:982-6. [Crossref] [PubMed]
- da Costa KA, Malvezzi H, Viana BG, et al. Validation of Laser Capture Microdissection Protocol in Endometriosis Studies. Medicina (Kaunas) 2019;55:520. [Crossref] [PubMed]
- Laser Capture Microdissection system | UseScience. Available online: https://scientificservices.eu/item/laser-capture-microdissection-system/1891 (accessed 28 February 2022).
- Malapelle U, de Rosa N, Rocco D, et al. EGFR and KRAS mutations detection on lung cancer liquid-based cytology: a pilot study. J Clin Pathol 2012;65:87-91. [Crossref] [PubMed]
- Selbach L, Kowalski T, Gerwert K, et al. Shape decomposition algorithms for laser capture microdissection. Algorithms Mol Biol 2021;16:15. [Crossref] [PubMed]
- Liotta LA, Pappalardo PA, Carpino A, et al. Laser Capture Proteomics: spatial tissue molecular profiling from the bench to personalized medicine. Expert Rev Proteomics 2021;18:845-61. [Crossref] [PubMed]
- Dong X, Shi M, Lee M, et al. Global, integrated analysis of methylomes and transcriptomes from laser capture microdissected bronchial and alveolar cells in human lung. Epigenetics 2018;13:264-74. [Crossref] [PubMed]
- Roy Chowdhuri S, Hanson J, Cheng J, et al. Semiautomated laser capture microdissection of lung adenocarcinoma cytology samples. Acta Cytol 2012;56:622-31. [Crossref] [PubMed]
- Ong CJ, Tan QX, Lim HJ, et al. An Optimised Protocol Harnessing Laser Capture Microdissection for Transcriptomic Analysis on Matched Primary and Metastatic Colorectal Tumours. Sci Rep 2020;10:682. [Crossref] [PubMed]
- Ayars M, Goggins M. Pancreatic cancer: Classifying pancreatic cancer using gene expression profiling. Nat Rev Gastroenterol Hepatol 2015;12:613-4. [Crossref] [PubMed]
- Grafen M, Hofmann TR, Scheel AH, et al. Optimized expression-based microdissection of formalin-fixed lung cancer tissue. Lab Invest 2017;97:863-72. [Crossref] [PubMed]
- Selamat SA, Chung BS, Girard L, et al. Genome-scale analysis of DNA methylation in lung adenocarcinoma and integration with mRNA expression. Genome Res 2012;22:1197-211. [Crossref] [PubMed]
- Huang P, Kong Q, Gao W, et al. Spatial proteome profiling by immunohistochemistry-based laser capture microdissection and data-independent acquisition proteomics. Anal Chim Acta 2020;1127:140-8. [Crossref] [PubMed]
- Hanson JC, Tangrea MA, Kim S, et al. Expression microdissection adapted to commercial laser dissection instruments. Nat Protoc 2011;6:457-67. [Crossref] [PubMed]
- Tangrea MA, Chuaqui RF, Gillespie JW, et al. Expression microdissection: operator-independent retrieval of cells for molecular profiling. Diagn Mol Pathol 2004;13:207-12. [Crossref] [PubMed]
- Hipp J, Cheng J, Hanson JC, et al. SIVQ-aided laser capture microdissection: A tool for high-throughput expression profiling. J Pathol Inform 2011;2:19. [Crossref] [PubMed]
- Tangrea MA, Mukherjee S, Gao B, et al. Effect of immunohistochemistry on molecular analysis of tissue samples: implications for microdissection technologies. J Histochem Cytochem 2011;59:591-600. [Crossref] [PubMed]
- Coope RJ, Schlosser C, Corbett RD, et al. Whole-slide laser microdissection for tumour enrichment. J Pathol 2021;253:225-33. [Crossref] [PubMed]
- Großerueschkamp F, Bracht T, Diehl HC, et al. Spatial and molecular resolution of diffuse malignant mesothelioma heterogeneity by integrating label-free FTIR imaging, laser capture microdissection and proteomics. Sci Rep 2017;7:44829. [Crossref] [PubMed]
- Großerueschkamp F, Kallenbach-Thieltges A, Behrens T, et al. Marker-free automated histopathological annotation of lung tumour subtypes by FTIR imaging. Analyst 2015;140:2114-20. [Crossref] [PubMed]
- Herrera JA, Mallikarjun V, Rosini S, et al. Laser capture microdissection coupled mass spectrometry (LCM-MS) for spatially resolved analysis of formalin-fixed and stained human lung tissues. Clin Proteomics 2020;17:24. [Crossref] [PubMed]
- Chen B, Kwan KY. Patterning and Cell Type Specification in the Developing CNS and PNS: Comprehensive Developmental Neuroscience. Academic Press, 2020.
- Freeman WM, Hemby SE. Proteomics for protein expression profiling in neuroscience. Neurochem Res 2004;29:1065-81. [Crossref] [PubMed]
- Mickler EA, Zhou H, Phang TL, et al. Low-Coverage Whole Genome Sequencing Using Laser Capture Microscopy with Combined Digital Droplet PCR: An Effective Tool to Study Copy Number and Kras Mutations in Early Lung Adenocarcinoma Development. Int J Mol Sci 2021;22:12034. [Crossref] [PubMed]
- Eckert MA, Coscia F, Chryplewicz A, et al. Proteomics reveals NNMT as a master metabolic regulator of cancer-associated fibroblasts. Nature 2019;569:723-8. [Crossref] [PubMed]
- Wiśniewski JR, Ostasiewicz P, Duś K, et al. Extensive quantitative remodeling of the proteome between normal colon tissue and adenocarcinoma. Mol Syst Biol 2012;8:611. [Crossref] [PubMed]
- Wang H, Qian WJ, Mottaz HM, et al. Development and evaluation of a micro- and nanoscale proteomic sample preparation method. J Proteome Res 2005;4:2397-403. [Crossref] [PubMed]
- Hughes CS, McConechy MK, Cochrane DR, et al. Quantitative Profiling of Single Formalin Fixed Tumour Sections: proteomics for translational research. Sci Rep 2016;6:34949. [Crossref] [PubMed]
- Pierobon M, Baldelli E, Hodge KA, et al. Development of a quantitative PD-L1 assay using laser capture microdissection (LCM)-based reverse phase protein microarray (RPPA) workflow: Implications for precision medicine. J Clin Orthod 2018;36:35. [Crossref]
- Vu QD, Graham S, Kurc T, et al. Methods for Segmentation and Classification of Digital Microscopy Tissue Images. Front Bioeng Biotechnol 2019;7:53. [Crossref] [PubMed]
- Wang S, Rong R, Yang DM, et al. Computational Staining of Pathology Images to Study the Tumor Microenvironment in Lung Cancer. Cancer Res 2020;80:2056-66. [Crossref] [PubMed]
- Mueller C, Davis JB, Liotta LA. Combining the "Sibling Technologies" of Laser Capture Microdissection and Reverse Phase Protein Microarrays. Adv Exp Med Biol 2019;1188:95-111. [Crossref] [PubMed]
Cite this article as: Seminati D, Casati G, Pagni F, Fraggetta F. Laser capture microdissection in lung cancer: a narrative review. J Xiangya Med 2022;7:8.


