
The role of the A0 pulley in trigger finger: a cadaver model
Introduction
Trigger finger is a common and often debilitating condition of the digits caused by impaired gliding of flexor tendons within the flexor sheath. Out of five reported retinacular annular pulleys in the digits, trigger finger has been reported to involve the first annular retinacular pulley (A1 pulley) most commonly (1). As such, trigger finger symptoms are usually treated via surgical A1 pulley release. Anecdotally, however, we have found that isolated A1 release often results in inadequate symptom resolution and/or recurrence. This may be due to constrictive contributions from alternative structures, as there are reports of the palmar aponeurosis (PA) or “A0” pulley being responsible for trigger finger in adults (2,3), and a recent clinical trial at our institution implicated the A0 pulley as a primary source of triggering in 31% to 47% of patients (4). Better understanding of these biomechanical relationships may help improve the overall treatment for patients suffering with trigger finger.
An experiment done in 2013 by Liu et al. created an accurate cadaveric hand model of trigger finger using a simple cable tie to apply increasing circumferential force to flexor tendons at the A1, A2, A3, and A4 pulleys, thereby simulating pulley constriction (5). While this study demonstrated that constriction at the site of the A1 pulley, but not the A2, A3, or A4, in the thumb, index, middle, and ring fingers was sufficient to induce triggering, it did not examine if constriction of the A0 pulley was sufficient to induce triggering (6-8). Additionally, no studies have adequately tested the contributions of the flexor digitorum profundus (FDP) and flexor digitorum superficialis (FDS) tendons by pulling on the FDP and FDS separately, simulating genuine hand grips in trigger finger.
The specific aims of this study were to simulate triggering with either A1 or A0 pulley constriction using a similar cadaver model, and as a secondary aim, to apply differential tension to the FDS while measuring tension in the FDP to simulate in vivo finger flexion during triggering. We hypothesized that constriction of the A0 pulley would be sufficient to induce finger triggering in a cadaver model. Importantly, our use of the term A0 pulley in this study refers to an individual pulley just proximal to the A1 pulley. While this pulley is typically referred to simply as the PA pulley and depicted as having an attachment to the transverse fibers of the PA (9), we have observed through cadaver dissections that the PA pulley does not have any attachments to the PA. Thus, while Kang et al. defined the transverse carpal ligament as an A0 pulley, we believe the PA pulley is a true A0 pulley (10). The data presented in the following article is in accordance with the MDAR reporting checklist (available at https://dx.doi.org/10.21037/jxym-21-21).
Methods
Hand procurement
Two left upper extremities, cleanly harvested above the elbow and flash frozen were procured from Science Care, Inc. (Phoenix, AZ, USA). The study was deemed IRB exempt by the Yale Institutional Review Board and was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Hand preparation
Hands were equilibrated at room temperature for 4 hours before manipulation. Kirschner wires (1.6 mm) were drilled through the metacarpals of the index, middle, ring, and small fingers to create a cradle for mounting on our computer-integrated tensiometer (Instron High Precision Soft Tissue Testing System, Model 5848; Instron Corporation, Norwood, MA, USA)
The FDP and FDS were dissected proximal to the carpal tunnel for individual flexion of the digits. A loop of 0 braided polyester suture was sutured to the end of each FDP to anchor the tendon to the load cell of the tensiometer. A loop of 2-0 braided polyester suture was sutured to the end of each FDS to attach to the manual Berkley Digital Scale 20 kg. The arm was mounted on a wooden block using the drilled K-wires and the apparatus was secured to the tensiometer’s lower base (Figure S1).
Cable tie and tension calculations
The A1 and A0 pulleys were exposed via Bruner incisions. Cables ties were threaded deep to the volar plate around the A1 pulley to create constriction, mimicking the anatomical pulley. For the A0 pulley, cable ties were threaded deep to the extensor tendons to be flush against the metacarpal.
The formulas and procedures for tensions applied by the cable tie on the flexor tendons were taken from Liu et al. (5). The cable ties (Commercial Electric, Cleveland, OH, USA) were made of nylon, measuring 4.7±0.02 mm in width and 1.4±0.02 mm in thickness. The track and ratchet mechanism was shortened by 1 mm and locked with each successive click. The nylon ties were sufficiently thin and flexible to allow threading around the pulley system and were of an appropriate width to replicate the length of an average A1 pulley, which, as determined by a previous study, measured 6.1±0.17 mm averaged across all fingers (5).
To determine the force of constriction of the cable ties on the tendons, a free cable tie was attached to the digital scale. The cable tie was pulled through until the circumference was 40 mm. Then, the tension necessary for advancing the cable tie for each click was measured until a circumference of 9 mm, smaller than the circumference of any tendon. This was repeated over three different free ties and the values were averaged. We control for the baseline tension delivered directly to the tendons by subtracting the tension necessary to advance the free-standing cable tie from the tension necessary to advance the cable tie on the cadaver model. Tension (the measured longitudinal force on the cable tie) was converted to a measure of radial compression force as per the thin-walled hoop stress theory using the following formula (5,11):
TNC, or tension normalized by circumference, is a measure of force proportional to the radial constriction force of the inner surface of the cable tie, and is therefore proportional to the pressure applied directly to the tendon. The minimal TNC necessary to induce triggering (mTNC) was extrapolated for each finger. This was calculated based on the initial tightening of the cable tie necessary to observe triggering via tensiometer output.
Trial preparation
Data were collected using the Instron High Precision Soft Tissue Testing System (Instron Corporation, Norwood, MA, USA), controlled by the BlueHill2 software (Instron Corporation, Norwood, MA). The hands were mounted vertically such that the fingers fell in to the position of natural digital cascade (Figure S1).
The FDP tendons were attached to the Instron tensiometer’s upper mobile arm via the suture loops. Movement of the mobile arm displaced the tendons upwards, simulating finger flexion. Each excursion was set to distract the tendon 40 mm/min for a total excursion of 50 mm. Simultaneous to each excursion, the FDS was attached via the suture loop to a manual tensiometer.
Initial excursions of the FDP without dissection yielded a maximum force of flexion of approximately 10 N. Based on Vigoroux’s estimation of the force ratios between the FDS and FDP tendons, as described above, and assuming a constant force of 10 N on the FDP, we calculated that the force applied to the FDS should be roughly 5.7 N for the crimp grip and 11 N for the slope grip, where the crimp grip refers to gripping a small edge with the proximal interphalangeal (PIP) joint flexed 90° to 100° and the distal interphalangeal (DIP) joint hyper-extended and the slope grip refers to a wide grasp with the PIP joint slightly flexed and the DIP joint flexed 50° to 70° (12). To simplify, we aimed to apply 5 and 10 N manually to the FDS to simulate in-vivo grip styles.
Three FDS conditions were conducted for each cable tie constriction, one with the FDS untensioned, one with 5 N manual tension, and one with 10 N to simulate the differential movements of the FDP and FDS relative to one another. Each condition was repeated three times while recording the load excursion. After each excursion, the fingers were returned to their initial degree of extension. The nine excursions (three for each FDS condition) were referred to as one trial.
Following initial trial runs in the tensiometer, a cable tie was tightened to a starting circumference of 40 mm around the A1 pulley. The hand-held tensiometer was attached to the free end of the cable tie, and the force used to tighten the pulley by 1 click was measured. A full flexion trial was conducted using the tensiometer to measure work. Full flexion trials were repeated for each cable tie click until the cable mounting tie could not be tightened further, with care taken not to shred or deform the underlying tendon. The cable tie was removed by cutting the loop portion with a scalpel, also taking care not to damage tissue. Excursions of each digit were reset to the same level of extension and terminated at the same point of flexion to provide consistent parameters of measurement to compare work of flexion (WOF). New cable ties were then threaded and this was repeated for the A0 pulley on each digit.
Data collection
The WOF was calculated as the integral of the load versus excursion curve for full digital flexion. Trials of each digit were reset to the same level of extension and terminated at the same point of flexion to provide consistent parameters of measurement (Figure S1). The maximum load sustained by the FDP, represented by the peak on the Y axis, was recorded for each excursion and averaged over all three trials. This maximal WOF was compared between triggering at the mTNC and the non-triggering condition at the immediate lower TNC.
Measurement of trigger magnitude
ImageJ 2.0.0 software was used to measure the magnitude of triggering. A direct vertical measurement was taken from the peak height immediately preceding triggering to the trough height of following the trigger. Measurements were standardized against the y-axis scale in Newtons. Measures were taken for each individual trial.
Statistical analysis
All values reported are averages from the 3 performed excursions for each study condition (3 excursions per finger, per hand). No data points were removed or excluded. One-way repeated measures ANOVA testing was performed for comparisons of WOF, maximal force of flexion, and magnitude of triggering across the 3 different levels of FDS tension. Paired t-tests were used to compare the maximal force of flexion at mTNC (trigger pathology) and next immediate non-triggering TNC. Given the limited sample size of the study, distribution normality was assessed by combining measured values from all fingers, from which an assumption of normality for each individual finger was made. All statistical analysis was performed using STATA statistical software. P<0.05 was set as significant throughout. The data and code utilized in this study are not currently available on any public databases.
Results
mTNC
Triggering was demarcated by a sudden dip in the load excursion graph followed by a gradual increase and return to a normal curve (Figure 1). The mTNCs of Hand #1 are outlined in Table 1. It is noted that the mTNC was lower for the A0 than the A1 in both the small and ring fingers. No triggering was observed with constriction of the index A1, index A0, middle A1, and middle A0 in this specimen.
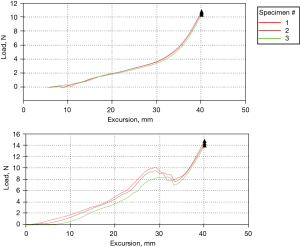
Table 1
| Finger tested | A1 | A0 |
|---|---|---|
| Hand 1 | ||
| Index | – | – |
| Middle | – | – |
| Ring | 0.63 | 0.01 |
| Small | 1.06 | 0.89 |
| Hand 2 | ||
| Index | 0.86 | 2.04 |
| Middle | 0.76 | 1.17 |
mTNC, minimum tension normalized by circumference.
In Hand #2, triggering was successfully induced in the index and middle fingers. The ring and small fingers were not tested in this hand due to accelerated degeneration of the fresh tissues in the warm laboratory environment, and the time required for testing of each digit. The mTNCs are outlined in Table 1. Of note, the mTNC was lower for both constriction of the A1 than the A0.
Impact of FDS tension of triggering at the mTNC
With constriction of the small A1 at the mTNC, triggering was elicited under all three FDS tensions. With small A0 constriction, no triggering was elicited with 0 N at the mTNC, but triggering resumed under 5 and 10 N tension.
With ring A1 constriction at the mTNC, triggering was elicited under all three FDS tensions. With ring A0 constriction at the mTNC, no triggering was elicited with 0N tension on the FDS, but triggering was elicited under 5 and 10 kg tension.
With middle A1 constriction at the mTNC, triggering occurred under all three FDS tensions. Likewise, with constriction of the middle A0 at the mTNC, triggering occurred under all three FDS tensions.
With index A1 constriction at the mTNC, triggering was elicited under all three FDS tensions. With A0 constriction at the mTNC, likewise, triggering was elicited under all three FDS tensions.
WOF at the mTNC
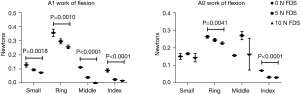
Throughout all fingers with A1 constriction at the mTNC, the WOF significantly decreased with increasing tension on the FDS (P=0.002, P=0.001, P<0.001, P<0.001; Figure 2). With A0 constriction, WOF significantly decreased with increasing FDS tension in the ring and index fingers (P=0.004; P<0.001), but no difference was seen in the small and middle.
Maximal force of flexion at the mTNC
In both small A0 and A1, increasing FDS tensions did not affect the maximal force of flexion at the mTNC. To ensure proper triggering, we compared the maximal force at the initial triggering condition with that of the immediately preceding constriction (Figure 3). With constriction of the A1 with 0 and 10 N of tension on the FDS, the difference in maximal force of flexion was significantly different between triggering and non-triggering conditions (P=0.018, P=0.019; Figure 4). With constriction of the A0, there was no difference in maximal force of flexion between triggering and non-triggering.


In both the index A1 and A0, increasing FDS tension significantly changed the maximal force of flexion at the mTNC (P=0.001; P<0.001). With constriction of the A1, the maximal force of flexion was significantly different between triggering and non-triggering in all three FDS conditions (P=0.004, P=0.001, P=0.002). With constriction of the A0, there was no difference between triggering and non-triggering.
In both middle A0 and A1, increasing FDS tension did not affect maximal force of flexion at the mTNC. With constriction of the A1, there was no difference between triggering and non-triggering. With constriction of the A0, the maximal force of flexion was significantly different between triggering and non-triggering in all three FDS conditions (P=0.025, P=0.041, P=0.001).
In both the index A1 and A0, increasing FDS tension significantly changed the maximal force of flexion at the mTNC (P<0.001; P=0.008). With constriction of the A1 and with the A0, the maximal force of flexion was significantly different between triggering and non-triggering in all FDS conditions (A1, P=0.013, P=0.004, P=0.046; A0, P=0.001, P=0.007, P=0.004).
Magnitude of triggering
The magnitude of triggering (Newtons) in the small A1 did not change significantly with increased FDS tension (Figure 5). The small A0 magnitude increased significantly with increased FDS tension (P=0.011). The ring A1 did not significantly change with FDS tension, while the ring A0 significantly increased (P<0.001). The middle A1 significantly decreased with increasing FDS tension (P<0.001), while the middle A0 increased but not significantly. The index A1 significantly decreased with increasing FDS tension (P=0.006), while the index A0 did not change significantly.

Discussion
Though trigger finger is traditionally associated with the A1 pulley (13), studies have suggested that other pulleys, notably the A2 and A3, can induce triggering (14-16). A recent study done by our group investigated this by creating a cadaveric model of trigger finger, utilizing a cable tie to apply increasing circumferential force on the flexor tendons while simultaneously tensioning the FDP (5). That study, however, did not examine the role of A0 pulley constriction in trigger finger (6-8). Secondly, in-vivo finger flexion is a coordinated effort between the FDP, FDS, and intrinsic hand muscles (17). Thus, our current study sought to (I) investigate the role of the A0 pulley in triggering and (II) to model in-vivo flexion by pulling on the FDP while sustaining the FDS under increasing amounts of fixed tension.
Our study demonstrated that constriction of the A0 pulley, otherwise known in literature as the PA pulley, was sufficient to induce triggering in the ring and small fingers of specimen #1, and the index and middle fingers of specimen #2. Past reports in literature have documented the PA as a cause of trigger finger (3). One patient experienced persistent triggering after release of the ring A1 pulley, and upon more extensive dissection, the transverse fibers of the PA were found to be responsible. The second patient reported a “double-click” in the palm. She received A1 pulley release with improvement in middle finger locking but persistent triggering, requiring PA release. In a more recent and larger randomized trial, 8 of 17 patients receiving only A0 release demonstrated complete resolution of symptoms (4). Wilhelmi et al. identified surface landmarks for trigger finger release (18). The proximal extent of current recommended release includes the A0 pulley as we have described. It is possible that the reason that A0 trigger finger is rarely reported is simply that this pulley is commonly released as a routine part of surgery (19).
In the comparison of minimal radial force upon the flexor tendons necessary to induce triggering (mTNC), we found that the mTNC was lower for the A0 than the A1 in both the small and ring fingers of the first hand, while it was lower for the A1 than the A0 in the middle and index fingers of the second hand, suggesting that certain conditions, fingers, or individuals, have a propensity towards either A1 or A0 trigger finger. Our modern consensus of pulley pathology stems from a study done by Sbernardori et al., which used scanning and transmission electron microscopy to scrutinize the A1 pulleys (20). Normal pulleys had an amorphous extracellular matrix coating the inner layer, but pathologic pulleys had areas of extracellular matrix loss characterized by chondrocyte proliferation and type III collagen production. The theory proposes that repeated physical force and compression between the flexor tendon and the inner layer of the A1 pulley produces this fibrocartilaginous metaplasia (1). This mechanism is likely not unique to the A1 pulley, and may reflect A0 pathology as well.
While the discussion of pulley pathology is important, the A0 or A1 pulley constriction was our independent variable. This implies that the tendons themselves were responsible for the differences observed. The relationship of the flexor tendons has been long studied in the pathogenesis of trigger finger. Wolfe described a fixed flexion deformity due to degenerative enlargement of the FDS that restricted both flexor tendon excursion through the A1 pulley (21). Trigger finger in children has been speculated to be due to an abnormal relationship between the FDP and FDS or a proximal decussation of the FDS (22).
Here, we have shown that this relationship is directly involved in the manifestation, maximal force, and magnitude of triggering. Without FDS tension in the small and ring fingers, triggering did not occur, while contrarily, with full FDS tension in the middle finger, triggering also did not occur. Maximal force of flexion was significantly different in the small A1, ring A1, middle A0, and index A1 and A0, consequently the same conditions that triggered at all mTNC FDS tensions. This suggests that triggering despite FDS tension may necessitate a large change in tendon dynamics while triggering dependent upon FDS flexion may be gradual and possibly less severe. We observed that increasing FDS tension significantly increased magnitude of triggering in the A0 of the small and ring fingers and significantly decreased triggering in the A1 of the middle finger and index finger. It is clear, then, that differential movements of the FDS and FDP work together to enhance trigger finger.
Our differential FDP and FDS tensions represented different hand grips. Reports have linked trigger finger to occupations requiring extensive gripping and finger flexion (23,24), a relationship now considered untrue (25). Our experiment also brings into question the role of hand grip in trigger finger. It is possible that certain static, incidence-only hand grips may elicit trigger finger in patients already with the pathology. This makes no comment on the origin or development of the tendon-sheath mismatch but, instead, states that the crimp grip or the slope grip may be implicated in inducing triggering in patients with trigger finger, depending on the individual, finger, or circumstance. WOF also decreased with increasing FDS tension with A1 constriction but not was not always the case with A0 constriction. The FDS and FDP may flex in concert as normal, during A1 trigger finger. During A0 trigger finger, perhaps tendons may even work against one another, causing increased flexion difficulties.
Since open or percutaneous surgical release is the definitive treatment for trigger finger, our study brings into question the utility of directed A1 release (26). Manske and Lesker noted that the total range of motion loss, with division of two pulleys, was the lowest with the A1 and PA together (2.8%) (2). In the face of persistent trigger finger pain and limitation, a 2.8% loss in flexion may be acceptable. Therefore, we recommend release of both A1 and A0 pulleys, in agreement with Wilhelmi et al. surface landmarks (18).
There are many limitations to this preliminary study. Although we were able to collect useful data points from these hands, individual variation exists, as exemplified by the lack of triggering in the middle and index finger in Hand #1. Furthermore, though we tried to replicate in-vivo flexion by applying a constant force to the FDS while pulling on the FDP, true flexion involves concerted action of the FDP, FDS, and intrinsic muscles. Most importantly, we understand that this study was limited by the use of only two cadaveric hands, and adequate power would require multiple other hands. While this limits broad conclusions, this experiment served as a preliminary survey of the A0 pulley in trigger finger, with findings to support that it can biomechanically be involved in trigger finger.
Conclusions
The results of this preliminary cadaver study indicate that trigger finger may arise from a delicate three-dimensional interplay between both the A0 and A1 pulleys, as well as the FDS and FDP tendons. These biomechanical relationships may vary between individuals and individual digits, and in response to various grip positions. Future studies should seek to better define the various anatomical contributions to trigger finger pathology in order to guide more effective treatment strategies.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the MDAR reporting checklist. Available at https://dx.doi.org/10.21037/jxym-21-21
Data Sharing Statement: Available at https://dx.doi.org/10.21037/jxym-21-21
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://dx.doi.org/10.21037/jxym-21-21). JGT serves as an unpaid editorial board member of Journal of Xiangya Medicine from December 2020 to November 2022. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sampson SP, Badalamente MA, Hurst LC, et al. Pathobiology of the human A1 pulley in trigger finger. J Hand Surg Am 1991;16:714-21. [Crossref] [PubMed]
- Manske PR, Lesker PA. Palmar aponeurosis pulley. J Hand Surg Am 1983;8:259-63. [Crossref] [PubMed]
- Sherman PJ, Lane LB. The palmar aponeurosis pulley as a cause of trigger finger. A report of two cases. J Bone Joint Surg Am 1996;78:1753-4. [Crossref] [PubMed]
- Wu RT, Walker ME, Peck CJ, et al. Differential Pulley Release in Trigger Finger: A Prospective, Randomized Clinical Trial. Hand (N Y) 2021; [Crossref] [PubMed]
- Liu KJ, Thomson JG. Experimental model of trigger finger through A1 pulley constriction in a human cadaveric hand: a pilot study. J Hand Surg Am 2013;38:1933-40. [Crossref] [PubMed]
- Chin KF, Khalid M, Gogi N, et al. The mend of the bend-flexor pollicis longus tendon has an additional pulley distal to its point of angulation. Clin Anat 2008;21:427-32. [Crossref] [PubMed]
- Marek DJ, Fitoussi F, Bohn DC, et al. Surgical release of the pediatric trigger thumb. J Hand Surg Am 2011;36:647-652.e2. [Crossref] [PubMed]
- Moutet F. Flexor tendon pulley system: anatomy, pathology, treatment. Chir Main 2003;22:1-12. [Crossref] [PubMed]
- Doyle JR. Anatomy and function of the palmar aponeurosis pulley. J Hand Surg Am 1990;15:78-82. [Crossref] [PubMed]
- Kang HJ, Lee SG, Phillips CS, et al. Biomechanical changes of cadaveric finger flexion: the effect of wrist position and of the transverse carpal ligament and palmar and forearm fasciae. J Hand Surg Am 1996;21:963-8. [Crossref] [PubMed]
- Gere JM, Timoshenko SP. Mechanics of Materials. 4th edition. Boston: PWS Pub Co, 1997.
- Vigouroux L, Quaine F, Labarre-Vila A, et al. Estimation of finger muscle tendon tensions and pulley forces during specific sport-climbing grip techniques. J Biomech 2006;39:2583-92. [Crossref] [PubMed]
- Makkouk AH, Oetgen ME, Swigart CR, et al. Trigger finger: etiology, evaluation, and treatment. Curr Rev Musculoskelet Med 2008;1:92-6. [Crossref] [PubMed]
- Walbeehm ET, McGrouther DA. An anatomical study of the mechanical interactions of flexor digitorum superficialis and profundus and the flexor tendon sheath in zone 2. J Hand Surg Br 1995;20:269-80. [Crossref] [PubMed]
- Tanaka T, Amadio PC, Zhao C, et al. The effect of partial A2 pulley excision on gliding resistance and pulley strength in vitro. J Hand Surg Am 2004;29:877-83. [Crossref] [PubMed]
- Nagaoka M, Yamaguchi T, Nagao S. Triggering at the distal A2 pulley. J Hand Surg Eur Vol 2007;32:210-3. [Crossref] [PubMed]
- Li ZM, Zatsiorsky VM, Latash ML. Contribution of the extrinsic and intrinsic hand muscles to the moments in finger joints. Clin Biomech (Bristol, Avon) 2000;15:203-11. [Crossref] [PubMed]
- Wilhelmi BJ, Mowlavi A, Neumeister MW, et al. Safe treatment of trigger finger with longitudinal and transverse landmarks: an anatomic study of the border fingers for percutaneous release. Plast Reconstr Surg 2003;112:993-9. [Crossref] [PubMed]
- Wilhelmi BJ, Snyder N 4th, Verbesey JE, et al. Trigger finger release with hand surface landmark ratios: an anatomic and clinical study. Plast Reconstr Surg 2001;108:908-15. [Crossref] [PubMed]
- Sbernardori MC, Mazzarello V, Tranquilli-Leali P. Scanning electron microscopic findings of the gliding surface of the A1 pulley in trigger fingers and thumbs. J Hand Surg Eur Vol 2007;32:384-7. [Crossref] [PubMed]
- Hotchkiss R. Elbow contracture. In: Boyer MI. Green’s operative hand surgery. 4th edition. Philadelphia, USA: Churchill Livingstone, 1999;1:667-82.
- Wood VE, Sicilia M. Congenital trigger digit. Clin Orthop Relat Res 1992;205-9. [PubMed]
- Gorsche R, Wiley JP, Renger R, et al. Prevalence and incidence of stenosing flexor tenosynovitis (trigger finger) in a meat-packing plant. J Occup Environ Med 1998;40:556-60. [Crossref] [PubMed]
- Bonnici AV, Spencer JD. A survey of 'trigger finger' in adults. J Hand Surg Br 1988;13:202-3. [Crossref] [PubMed]
- Trezies AJ, Lyons AR, Fielding K, et al. Is occupation an aetiological factor in the development of trigger finger? J Hand Surg Br 1998;23:539-40. [Crossref] [PubMed]
- Lin CJ, Huang HK, Wang ST, et al. Open versus percutaneous release for trigger digits: Reversal between short-term and long-term outcomes. J Chin Med Assoc 2016;79:340-4. [Crossref] [PubMed]
Cite this article as: Wu RT, Peck CJ, Gary CS, Kanouzi J, Persing JS, Thomson JG. The role of the A0 pulley in trigger finger: a cadaver model. J Xiangya Med 2021;6:31.


