
Three coronavirus disease 2019 cases with acute progression of respiratory failure were improved after methylprednisolone therapy
Introduction
An outbreak of viral pneumonia termed coronavirus disease 2019 (COVID-19), the causative agent of which is the novel pathogen severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), was first reported in Wuhan, China in December 2019. Following this, the illness rapidly spread globally (1). Development of acute respiratory distress syndrome (ARDS) is an important risk factor for acute death in patients with COVID-19 (1-3). Wu et al. found that 44 of 84 (52.4%) patients who developed ARDS attributable to COVID-19 died (2). SARS-CoV-2 infection is believed to dampen antiviral interferon responses via endosomal RNA receptors, such as Toll-like receptor 3 (TLR3), TLR7, and retinoic acid-inducible gene-I/melanoma differentiation-associated gene 5, resulting in uncontrolled viral replication and hyperproduction of pro-inflammatory cytokines (4). This insufficiency of the innate immune response may cause cytokine storms and promote the development of ARDS in patients with COVID-19. Recently, it was suggested that rare putative loss-of-function variants of X-chromosomal TLR7 that are associated with impairment of type I and II interferon responses were identified in severe COVID-19 patients (5). Immunosuppressive therapy had been considered to prevent cytokine storms and deterioration of COVID-19 (6), but treatment with corticosteroids (CS) against ARDS caused by SARS-CoV or Middle East respiratory syndrome (MERS-CoV) has not been recommended because of delayed clearance of virus RNA from respiratory tract secretions (7). A meta-analysis that was dominated by observational studies suggested that CS therapy for SARS-CoV-2, SARS-CoV, and MERS-CoV infections resulted in delayed virus clearance and did not improve survival, hospitalization duration, or the intensive care unit admission rate and/or mechanical ventilator use (8). However, some studies suggested that the time to SARS-CoV-2 RNA clearance was not different between patients treated with or without CS therapy (9,10). The RECOVERY trial, a controlled, open-label trial, compared mortality rates between patients with COVID-19 who received oral or intravenous dexamethasone (DEX, at a dose of 6 mg once daily) for up to 10 days or usual care alone. The primary outcome, the 28-day mortality rate, was lower among patients who received DEX compared with those who received usual care only (11). Treatment with anti-inflammatory drug candidates other than CS, including tocilizumab, hydroxychloroquine, and azithromycin has not improved mortality of COVID-19 cases in clinical trials (12,13). To date, the only drugs with evidence of efficacy against COVID-19 are CS. This fact contrasts with the results of a meta-analysis among patients with influenza, showing that CS increased mortality (14). One of the conveniences of DEX therapy compared with other CS is its lower cost. However, the efficacy of CS therapy other than DEX for COVID-19 has not been sufficiently clarified by clinical trial. In addition, it is necessary to discuss the optimal drug selection, timing of intervention, dose, and duration of CS therapy for COVID-19. We present three cases who suffered rapid respiratory failure progression due to COVID-19 but improved after methylprednisolone (MPSL) and discuss these issues in the present case report.
We obtained written informed consent for publication from the patients and received specific approval for all procedures from the Institutional Review Board of our hospital, in accordance with the ethical standards of the Helsinki Declaration of 2013 for therapy without insurance. We present the following case in accordance with the CARE reporting checklist (available at http://dx.doi.org/10.21037/jxym-21-5).
Case presentation
Case 1
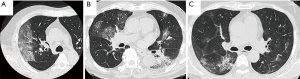
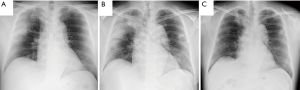
A 56-year-old Japanese man visited our hospital with a cough and fever of over 38 °C over three days. He was suspected of having COVID-19 pneumonia and was admitted to our hospital. Chest high resolution computed tomography (HRCT) images at admission of this patient and the following two cases reported herein are shown in Figure 1. Chest HRCT images revealed ground-glass attenuation (GGA) in the right lung. At this time, we started treatment with hydroxychloroquine (400 mg/day) and azithromycin (500 mg/day) after admission. On day 3 after admission, he was officially diagnosed with COVID-19 pneumonia based on a positive result from the third of triplicate SARS-CoV-2 real-time polymerase10’ chain reaction (RT-PCR) assays. The patient’s complications included hypertension and diabetes mellitus (DM), and those diseases activity were stabilized by receiving drug therapy or diet remedy. He had a smoking history of 16 packs/year. The characteristics and outcomes of this patient and the two following cases are shown in Table 1. This patient’s vital signs on admission were as follows: respiratory rate, 18 breaths/min; SpO2, 98% (room air); heart rate, 90/min; blood pressure, 114/65 mmHg; and body temperature, 38.8 °C. Auscultation revealed no abnormal respiratory or heart sounds. Hematology and other laboratory examinations showed elevated levels of C-reactive protein (CRP; 15.4 mg/dL), lactate dehydrogenase (LDH; 226.0 IU/mL), ferritin (1,066.6 pg/mL), and interleukin (IL)-6 (41.5 pg/mL). Change of chest radiograph in Case 1 is shown in Figure 2. On post-admission day 4, his condition deteriorated; inflammatory markers were elevated, and HRCT images revealed progressed pulmonary infiltration. He required oxygen inhalation of 5 L/min via a reservoir mask and received 500 mg/day of MPSL for 3 days followed by 30 mg/day of prednisolone (PSL) based on his diagnosis of COVID-19 with deterioration. After this therapy, his disease activity was improved, and both oxygen and CS therapy were discontinued on post-admission days 9 and 12, respectively. No serious side effects due to CS were observed. We confirmed that the results from RT-PCR assays for SARS-CoV-2 were negative on post-admission days 11 and 13. On day 14, we discontinued treatment with hydroxychloroquine, and the patient was discharged.
Full table
Case 2
An 84-year-old Japanese man presented to his local hospital 13 days after his son’s onset of COVID-19 with a fever of 38.3 °C and chills. Chest HRCT images showed GGA in the right lung, and laboratory findings showed elevated inflammation markers, so he was suspected of having COVID-19 and was admitted to our hospital. On post-admission day 2, he was officially diagnosed with COVID-19 pneumonia based on the SARS-CoV-2 RT-PCR results from a sputum sample. The patient’s complications included hypertension and DM, and those diseases activity were stabilized by receiving medical therapy or diet remedy. He had a smoking history of 36 packs/year. His vital signs on admission were as follows: respiratory rate, 16 breaths/min; SpO2, 98% (room air); heart rate, 64 beats/min; blood pressure, 144/79 mmHg; and body temperature, 38.3°C. Auscultation revealed no abnormal respiratory or heart sounds. Hematology and other laboratory examinations showed a slight increase in the levels of CRP (5.9 mg/dL), LDH (187.0 IU/mL), ferritin (371.8 pg/mL), and IL-6 (17.5 pg/mL). On day 2, we started treatment with hydroxychloroquine (400 mg/day) and azithromycin (500 mg/day). On day 4, the patient’s oxygen saturation decreased to 91%, and pulmonary infiltration on HRCT progressed to consolidations in both lungs, so he was treated with 500 mg/day of MPSL for 3 days. After this therapy, his disease activity was improved, and CS therapy and oxygen therapy were discontinued on post-admission days 6 and 9, respectively. No serious side effects due to CS were observed. After RT-PCR testing for SARS-CoV-2 produced negative results on days 9 and 10, we discontinued treatment with hydroxychloroquine, and the patient was discharged on post-admission day 10.
Case 3
A 49-year-old Japanese man visited our hospital because of general fatigue, dyspnea, and a fever of over 38 °C that had lasted for 8 days. HRCT images showed patchy GGA with consolidation in the bilateral lungs. The patient’s complications included DM, hypertension, chronic kidney disease, and an old cerebral infarction, and receiving medical therapy for DM and hypertension, and those diseases activity were stabilized by receiving drug therapy or diet remedy. He reported that he had never smoked. His vital signs on admission were as follows: SpO2, 93% at 5 L/min of O2 by nasal cannula; heart rate, 111 beats/min; blood pressure, 161/100 mmHg; and body temperature, 38.6 °C. Auscultation revealed no abnormal respiratory or heart sounds. Hematology and other laboratory examinations showed a slight increase in the levels of CRP (10.7 mg/dL), LDH (502.0 IU/mL), ferritin (1134.2 pg/mL), and IL-6 (9.0 pg/mL). COVID-19 was suspected, and we started treatment with hydroxychloroquine (400 mg/day) and azithromycin (500 mg/day). On day 2, his SARS-CoV-2 RT-PCR results were positive. The same day, the patient’s oxygenation was further reduced, so he received 500 mg/day of MPSL followed by 40 mg/day of PSL with a subsequent taper. His oxygenation improved remarkably, and the oxygen and CS therapy were both discontinued on post-admission day 12. On post-admission day 19, we discontinued treatment with hydroxychloroquine, and the patient was relocated to another hospital to continue treatment. No serious side effects due to CS for COVID-19 were observed. After RT-PCR testing for SARS-CoV-2 produced negative results on post-admission days 23 and 25, the patient was discharged.
Discussion
In the present case series, patients were treated with MPSL but not DEX, which improved mortality of COVID-19 patients in the RECOVERY trial. Most ongoing trials of CS therapies against COVID-19 suspended enrollment because benefit of DEX therapy was clarified in the RECOVERY trial (15). A meta-analysis of seven randomized controlled trials (RCTs) of CS therapy for COVID-19 demonstrated that CS, including MPSL, hydrocortisone (HC), and DEX, reduced mortality at 21, 28 or 30 days compared to usual care or placebo [odds ratio, 0.66; 95% confidence interval (CI), 0.53–0.82] (15). A phase IIb RCT suggested that short course MPSL therapy for COVID-19 patients reduced mortality in cases over 60 years of age but not in the overall population (10). In a retrospective study in Wuhan, MPSL therapy decreased the risk of death among patients with COVID-19-associated ARDS (hazard ratio, 0.38; 95% CI, 0.20–0.72) (2). Among 11 Japanese cases with COVID-19 who required oxygen inhalation in a previous report, 10 (91%) cases responded well to treatment with MPSL and favipiravir, requiring no further oxygen supplementation or ventilator management (16). Therefore, we believe that the efficacy of MPSL therapy for COVID-19 is reproducible and can be generalized to other populations. MPSL therapy has often been selected for severe respiratory failure empirically in clinical practice in Japan because of the reported therapeutic effects of 0.5–2.5 mg/kg of MPSL for ARDS patients of various etiologies (17,18). The difference in efficacy of between MPSL and other corticosteroids is unclear, as no prospective controlled studies compared efficacy of each corticosteroids. This issue should be analyzed in the further clinical trial.
The optimal timing of CS therapy intervention has not been established. In the RECOVERY trial, DEX therapy improved mortality among patients who received either invasive mechanical ventilation or oxygen alone at randomization, but not among patients receiving no respiratory support (11). Conversely, the meta-analysis of CS therapy for COVID-19 showed that patients who received mechanical ventilation or vasoactive medication tended to have lower mortality than those who did not (15). In a previous report of Japanese COVID-19 cases, treatment with MPSL and favipiravir was started when patients had a blood oxygen saturation ≤93% on room air or required oxygen inhalation (16). All three present cases were also started on MPSL therapy immediately when oxygen administration was required. Thus, CS therapy should be started in the early stage of respiratory failure. In addition, early intervention with CS is required in cases with aggravating risk factors such as older age, complicating DM, hypertension, or malignant diseases (2,3).
The optimal dose and duration of CS therapy has not been assessed in previous studies and higher dose CS therapy had not been proven to be more beneficial than lower dose CS therapy (15). The present cases were treated with 500 mg/day of MPSL for 3 days followed by case-specific maintenance PSL therapy for 3–11 days and no serious side effects due to CS that cause drug discontinuation were observed. In previous studies, COVID-19 patients were treated with 500 mg/day of MPSL for 3 days or 80 mg/day for 3–5 days (10,15,16). High-dose and long-term administration of CS can cause side effects such as infections. Meta-analysis of COVID-19 patients showed that administration of CS did not increase risk of serious of adverse events (15).
The combination therapies of inhibitors of virus replication such as favipiravir, remdesivir and CS, which are anti-inflammatory drugs, may show an efficient therapeutic effect on COVID-19, but this has not yet been sufficiently analyzed (16,19,20). Efficacy of these combination therapies as well as the successful case report of treatment with MPSL and favipiravir in a Japanese COVID-19 patient (16), should be analyzed in greater detail.
The major limitation of the present case report is the small number of successful cases of MPSL therapy. Larger studies are needed to clarify the efficacy and tolerability of MPSL therapy for COVID-19.
Conclusions
We report three cases who suffered rapid respiratory failure progression due to COVID-19 but were improved after MPSL treatment. MPSL is a candidate drug for CS therapy for COVID-19, which should be started in the early stage of respiratory failure. Optimal drug selection, timing of intervention, dose, and duration of CS therapy for COVID-19 should be analyzed in the further studies.
Acknowledgments
We thank Yasuhiro Kishihara and Tsuyoshi Ihara, as well as M.D. and Ph.D. in the Department of General Internal Medicine, National Hospital Organization Kyushu Medical Center (Miki Odawara, Motoko Fukamachi, Norihiro Arikawa, Kazuki Ogata, Kayoko Iwaya, and Mayuko Kishikawa, as well as nurse specialists and other medical staff of the infectious disease control team in the National Hospital Organization Kyushu Medical Center who participated in the therapy for the reported cases.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at http://dx.doi.org/10.21037/jxym-21-5
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jxym-21-5). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020;395:497-506. [Crossref] [PubMed]
- Wu C, Chen X, Cai Y, et al. Risk Factors Associated With Acute Respiratory Distress Syndrome and Death in Patients With Coronavirus Disease 2019 Pneumonia in Wuhan, China. JAMA Intern Med 2020;180:934-43. [Crossref] [PubMed]
- Wang D, Hu B, Hu C, et al. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA 2020;323:1061-9. [Crossref] [PubMed]
- Prompetchara E, Ketloy C, Palaga T. Immune responses in COVID-19 and potential vaccines: Lessons learned from SARS and MERS epidemic. Asian Pac J Allergy Immunol 2020;38:1-9. [PubMed]
- van der Made CI, Simons A, Schuurs-Hoeijmakers J, et al. Presence of Genetic Variants Among Young Men With Severe COVID-19. JAMA 2020;324:663-73. [Crossref] [PubMed]
- Mehta P, McAuley DF, Brown M, et al. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet 2020;395:1033-4. [Crossref] [PubMed]
- Russell CD, Millar JE, Baillie JK. Clinical evidence does not support corticosteroid treatment for 2019-nCoV lung injury. Lancet 2020;395:473-5. [Crossref] [PubMed]
- Li H, Chen C, Hu F, et al. Impact of corticosteroid therapy on outcomes of persons with SARS-CoV-2, SARS-CoV, or MERS-CoV infection: a systematic review and meta-analysis. Leukemia 2020;34:1503-11. [Crossref] [PubMed]
- Fang X, Mei Q, Yang T, et al. Low-dose corticosteroid therapy does not delay viral clearance in patients with COVID-19. J Infect 2020;81:147-78. [Crossref] [PubMed]
- Jeronimo CMP, Farias MEL, Val FFA, et al. Methylprednisolone as Adjunctive Therapy for Patients Hospitalized With COVID-19 (Metcovid): A Randomised, Double-Blind, Phase IIb, Placebo-Controlled Trial. Clin Infect Dis 2021;72:e373-81. [Crossref] [PubMed]
- RECOVERY Collaborative Group. Dexamethasone in Hospitalized Patients with Covid-19. N Engl J Med 2021;384:693-704. [Crossref] [PubMed]
- Furlow B. COVACTA trial raises questions about tocilizumab's benefit in COVID-19. Lancet Rheumatol 2020;2:e592 [Crossref] [PubMed]
- Horby P, Mafham M, Linsell L, et al. Effect of Hydroxychloroquine in Hospitalized Patients with Covid-19. N Engl J Med 2020;383:2030-40. [Crossref] [PubMed]
- Lansbury LE, Rodrigo C, Bee JL, et al. Corticosteroids as Adjunctive Therapy in the Treatment of Influenza: An Updated Cochrane Systematic Review and Meta-analysis. Meta-Analysis Crit Care Med 2020;48:e98-106. [Crossref] [PubMed]
- Sterne JAC, Murthy S, Diaz JV, et al. Association Between Administration of Systemic Corticosteroids and Mortality Among Critically Ill Patients With COVID-19: A Meta-analysis. JAMA 2020;324:1330-41. [Crossref] [PubMed]
- Murohashi K, Hagiwara E, Kitayama T, et al. Outcome of early-stage combination treatment with favipiravir and methylprednisolone for severe COVID-19 pneumonia: A report of 11 cases. Respir Investig 2020;58:430-4. [Crossref] [PubMed]
- Meduri GU, Golden E, Freire AX, et al. Methylprednisolone infusion in early severe ARDS: results of a randomized controlled trial. Chest 2007;131:954-63. [Crossref] [PubMed]
- Tang BM, Craig JC, Eslick GD, et al. Use of corticosteroids in acute lung injury and acute respiratory distress syndrome: a systematic review and meta-analysis. Crit Care Med 2009;37:1594-603. [Crossref] [PubMed]
- Grein J, Ohmagari N, Shin D, et al. Compassionate Use of Remdesivir for Patients With Severe Covid-19. N Engl J Med 2020;382:2327-36. [Crossref] [PubMed]
- Cai Q, Yang M, Liu D, et al. Experimental Treatment with Favipiravir for COVID-19: An Open-Label Control Study. Engineering (Beijing) 2020;6:1192-8. [Crossref] [PubMed]
Cite this article as: Fujiyoshi R, Okamoto M, Nagasaki Y, Tokunaga Y, Furuya K, Shimo M, Ishihara S, Takeoka H, Naitou-Nishida Y, Nouno T, Sakamoto S, Yamada H, Yano R, Iwasaki H, Hoshino T, Morita S. Three coronavirus disease 2019 cases with acute progression of respiratory failure were improved after methylprednisolone therapy. J Xiangya Med 2021;6:20.


