
De Winter sign associated with roes-induced anaphylactic shock: a new electrocardiographic manifestation of Kounis syndrome
Introduction
Kounis syndrome (KS) has been established as a coronary disorder caused by allergic, hypersensitivity, anaphylactic and anaphylactoid reactions. The de winter electrocardiogram (ECG) sign signifies critical proximal occlusion of left anterior descending artery (LAD), regarded as ST-elevation myocardial infarction (STEMI) equivalent pattern. This ECG pattern has high positive predictive value for acute occlusion of LAD. To our best knowledge, there has been no report about the feature of de Winter ECG pattern in patients with KS. In this article, we report a case of a patient with KS and anaphylactic shock presenting with a de Winter ECG pattern. We present the following article in accordance with the CARE reporting checklist (available at http://dx.doi.org/10.21037/jxym-20-119).
Case presentation
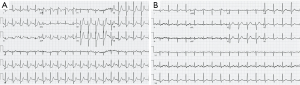
A 44-year-old male patient, smoker (a pack per day for 10 years), with history of hypersensitivity, was presented with skin pruritus after eating roes for an hour, and shortness of breath, no response to command for half an hour. His blood pressure was undetectable. Diffuse erythema was all over the body. No rales were found in the lungs. His heart rate was 115 beats per minute, and the heart rhythm was regular. The heart sound was weak, and no murmur was heard. An initial ECG showed upsloping ST-segment depression followed by tall, symmetrical T waves in leads II, aVF, and V2–V6, while lead aVR exhibited ST-segment elevation (Figure 1A). The patient was administrated with intravenous dexamethasone 10 mg, and subcutaneous adrenaline (1 mg/mL) 0.5 mg immediately. His blood pressure rose to 103/62 mmHg, and his response to command recovered quickly. Of note, the ECG findings were consistent with characteristics of de Winter ECG pattern. Therefore, serial ECG and cardiac makers were measured. An ECG performed 30 minutes after antiallergic treatment showed normalization of the T-wave amplitude in leads II, aVF, and V2 to V6, and resolution of all ST segment depressions (Figure 1B). The ECG showed the same performance after two hours. The serial values of creatine kinase MB (CK-MB) were normal, and the peak value of troponin T was 0.066 µg/L (normal range <0.013 µg/L). The patient was no chest pain and tightness, and discharged on the second day. According to our follow-up, the patient had no discomfort after discharge for 1 month. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient.
Discussion
As we known, KS is defined as the occurrence of acute coronary syndrome (ACS) associated with an anaphylactic reaction (1), which was first detailed reported and recorded by Kounis NG in 1991 (2). Usually, KS can be divided into three types, including coronary spasm (type I variant), coronary thrombosis (type II variant) and stent thrombosis (type III variant). In the type I KS, patients with normal coronary arteries in whom coronary artery spasm occurs due to the release of inflammatory mediators in an acute anaphylactic reaction and abnormal ion channel activation. In the type II KS, anaphylactic reaction dependent plaque erosion or rupture in patients with preexisting coronary atheromatous lesion leads to ACS. Type III KS involves patients with coronary artery stent thrombosis. Current researches suggest KS is caused by inflammatory mediators such as histamine, platelet-activating factor, arachidonic acid products, neutral proteases and a variety of cytokines and chemokines released during the allergic activation process. In detail, platelet activation and involving interrelated and interacting inflammatory cells participate KS.
Recently, increasing evidence suggest that KS could induce a similar picture of ST elevation in patients with and without coronary artery disease due to vasospastic action of the released anaphylactic mediators or due to mild inflammatory injury induced by the action of the released inflammatory mediators during anaphylaxis.
For example, a unique sign of ST elevation in lead aVR, with reciprocal ST depression in the majority of other leads has been described recently in KS (3). Furthermore, patients with history of hypersensitivity who developed KS could have ECG abnormalities and even with normal or slightly increased troponin (4). In present case, the slightly increase of myocardial injury markers revealed mild and transitory myocardial ischemic injury due to coronary artery spasm rather than acute coronary thrombosis which could induce a sharp increase in troponin and typical chest pain. The specific feature of ST-segment changes in the inferolateral leads may be associated with the LAD coronary artery spasm caused by hypersensitivity. In addition, the patient recovered quickly, and the specific feature of ECG disappeared after rapid intravenous dexamethasone treatment. This result could explain by the remission of LAD coronary spasm after antiallergic treatment. Combined with the patient’s clinical history, the patient proved to be the type I KS. It’s better to get the coronary imaging evidence of normal coronary artery to support our idea. However, coronary arteriography was not performed based on the beneficial effects of the administered drugs.
To date, de Winter sign was seldom reported in the patients without coronary artery disease. To our best knowledge, it is firstly reported that de Winter ECG pattern is present in the patient with KS. As we known, de Winter sign is regard as a sign of proximal LAD occlusion, which was first reported in 2008 by de Winter et al. in a group of patients with anterior myocardial infraction and this ECG pattern accounted for about 2% in symptomatic LAD artery occlusion (5). The typical de Winter ECG pattern is characterized by upsloping ST-segment depression at the J-point (>1 mm) followed by tall, symmetrical T-waves in the precordial leads, and a mean J-point elevation of approximately 0.5 mm in lead aVR leads. The de Winter ECG pattern has positive predictive values of 95% to 100% in the respective diagnostic studies (6). Non-LAD artery occlusion featured with de Winter ECG pattern in ACS has also been reported (7). The underlying mechanisms of de Winter ECG pattern are not completely known. Potential explanation includes an anatomical variant of the Purkinje fibers with endocardial conduction delay or lack of activation of sarcolemmal adenosine triphosphate (ATP)-sensitive potassium channels by ischemic ATP depletion. A slightly elevated troponin T indicated that there may be a mild inflammatory injury caused by hypersensitivity in cardiac myocytes. Whether cardiac inflammatory injury results in de Winter sign by acting on sarcolemmal potassium channels in the patient needs a further study to verify.
Our findings suggested that KS may have ECG characteristics similar to de Winter ECG pattern, and specificity of the pattern still need to be clarified further. Of note, KS should be excluded for the patients suffering anaphylaxis with de Winter sign by serial examinations of ECG and cardiac markers. In some special cases, timely coronary angiography is helpful for differential diagnosis. Ideally a coronary arteriography should have been performed before discharge or soon after discharge. The practitioners should make a correct diagnosis basing on a comprehensive clinical condition to bring the best benefits to patients.
Acknowledgments
Funding: The National Natural Science Foundation of China (NSFC) Grant (NO.81660054) provided funding support in writing up this article.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at http://dx.doi.org/10.21037/jxym-20-119
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jxym-20-119). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Kounis NG. Kounis syndrome: an update on epidemiology, pathogenesis, diagnosis and therapeutic management. Clin Chem Lab Med 2016;54:1545-59. [Crossref] [PubMed]
- Kounis NG, Zavras GM. Histamine-induced coronary artery spasm: the concept of allergic angina. Br J Clin Pract 1991;45:121-8. [PubMed]
- Kounis NG, Koniari I, Soufras GD, et al. Sugammadex-induced atropine-resistant bradycardia: clinical, pathophysiologic, and electrocardiographic considerations. JA Clin Rep 2020;6:31. [Crossref] [PubMed]
- Kounis NG. Kounis syndrome: a monster for the atopic patient. Cardiovasc Diagn Ther 2013;3:1-4. [PubMed]
- de Winter RJ, Verouden NJ, Wellens HJ, et al. A new ECG sign of proximal LAD occlusion. N Engl J Med 2008;359:2071-3. [Crossref] [PubMed]
- Morris NP, Body R. The De Winter ECG pattern: morphology and accuracy for diagnosing acute coronary occlusion: systematic review. Eur J Emerg Med 2017;24:236-42. [Crossref] [PubMed]
- Montero Cabezas JM, Karalis I, Schalij MJ. De Winter Electrocardiographic Pattern Related with a Non-Left Anterior Descending Coronary Artery Occlusion. Ann Noninvasive Electrocardiol 2016;21:526-8. [Crossref] [PubMed]
Cite this article as: Dai W, Jiang Z, Zhong G. De Winter sign associated with roes-induced anaphylactic shock: a new electrocardiographic manifestation of Kounis syndrome. J Xiangya Med 2021;6:9.


