
An alternative viewpoint for the cardioprotective effects of ischemic preconditioning
Ischemic preconditioning was originally described by Murry et al. in 1986 (1). They demonstrated that brief ischemic episodes before prolonged ischemia reduced infarct size by 75% in a canine model of ischemia and reperfusion. Despite the potent cardioprotective effects of ischemic preconditioning, its clinical translation remains to be seen because it needs to be implemented before starting prolonged ischemia. It is difficult to implement this except in cases of planned coronary artery bypass surgery or heart transplantation. On the other hand, the potent cardioprotective effects of preconditioning garnered sufficient interest regarding its mechanisms because these might help elucidate the key process in the progression to irreversible ischemic cell injury. The most popular mechanism of the cardioprotective effects of preconditioning is the release of various triggering molecules, such as autacoids, neurohormones, and cytokines, in response to brief episodes of ischemia and reperfusion. The release of such molecules induces phosphorylation/activation of protein kinases, which triggers the initiation of intracellular signal transduction cascades, such as the reperfusion injury salvage kinase system, resulting in the prevention of both mitochondrial permeability transition and resultant cell deaths (2). However, the precise mechanisms of the cardioprotective effects of preconditioning still remain to be investigated. Especially, there is no reasonable explanation, thus far, for the total loss of the cardioprotective effects of preconditioning when subsequent ischemia was prolonged to 3 hours (1).
Apart from the precise molecular mechanisms, a hint for solving the long-standing question seems to lie in another cardioprotective approach that is as potent as preconditioning: temporary contractile activity blockade during reperfusion using 2,3-butanedione monoxime (BDM). Schlack et al. demonstrated that BDM administration immediately before reperfusion reduced infarct size by 73% in a canine model of ischemia and reperfusion (3). The infarct-sparing effect of BDM has been attributed to the prevention of reperfusion-induced hypercontracture. In their experiments, 60-minute ischemia was used instead of 40-minute ischemia. Nevertheless, infarct-size reduction was as robust as that achieved by preconditioning. Both preconditioning and BDM treatment seem to provide the most potent cardioprotection in the in vivo canine model of ischemia and reperfusion. As BDM administration was performed immediately before reperfusion, its infarct-sparing effects can be attributed purely to the prevention of lethal reperfusion injury. Conversely, myocardial necrosis caused by ischemic injury can be estimated to be <30% of the total infarcted myocardium caused by 60-minute ischemia followed by reperfusion in the canines. Looking back to ischemic preconditioning, similar maximum cardioprotection, i.e., 75% infarct-size reduction, is observed after 40 minutes of ischemia followed by reperfusion in the canine hearts. This cannot be attributed to the prevention of myocardial necrosis caused by ischemic injury, which should be <30% of the total amount of myocardial necrosis in 40-minute ischemia. Instead, it might be reasonable to assume that this large infarct-sparing effect (75%) resulted from the prevention of lethal reperfusion injury as in the BDM-treated hearts. Preconditioning, however, seems unlikely to have such potent, direct cardioprotective effects against lethal reperfusion injury. If so, how are preconditioned hearts protected? One possible explanation is that lethal reperfusion injury is not prevented, but rather avoided in preconditioned hearts.
The cardioprotective effects of preconditioning were completely lost after a 3-hour ischemic period followed by reperfusion (1), as if an all-or-none mechanism of preconditioning effects was present. In certain specific situations, this may happen (Figure 1). This situation requires an assumption that preconditioning can extend the ischemia duration necessary for lethal reperfusion injury to occur after reperfusion. There is a lag period, which is the difference in the ischemia duration necessary for lethal reperfusion injury to occur after reperfusion between the control and preconditioned hearts. If reperfusion starts during the lag period, lethal reperfusion injury occurs in the control but not the preconditioned hearts because the ischemia duration is not long enough for lethal reperfusion injury to occur. Furthermore, the difference may become evident because lethal reperfusion injury—once it has occurred—may become more severe when reperfusion starts earlier (4-6). This means that lethal reperfusion injury may be near its maximum severity around its onset. On the other hand, if reperfusion starts after the lag period, the difference between the two scenarios can no longer be observed because the lethal reperfusion injury occurs in both hearts. Thus, whether preconditioning induces a >70% reduction or no reduction in infarct size may depend on the time to reperfusion.
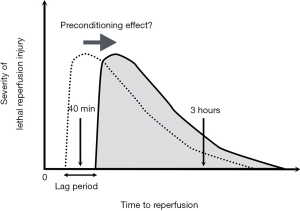
As mentioned earlier, ischemic preconditioning has triggered the investigation of its cardioprotective mechanism, seeking the key process in the progression to irreversible ischemic cell injury. However, if the cardioprotective effects of preconditioning reside in prolonging the ischemia duration necessary for lethal reperfusion injury to occur after reperfusion, the investigation should focus on the triggering mechanisms of lethal reperfusion injury rather than the key process in irreversible ischemic cell injury. In 1994, Schlack’s group, by using BDM, attempted to prevent reperfusion-induced hypercontracture (3), which develops with an elevated level of intracellular Ca2+ concentrations {[Ca2+]i} and re-energetization of myofilaments by adenosine triphosphate production after reperfusion, and demonstrated a marked infarct-size reduction in the canine model of ischemia and reperfusion. Years later, preconditioning has been demonstrated to delay the increase in [Ca2+]i during subsequent prolonged ischemia in rabbit pupillary muscle (7). Therefore, it may take longer for prolonged ischemia to achieve the threshold level of [Ca2+]i for developing hypercontracture of the myocardium after reperfusion in preconditioned hearts. Taken together, if reperfusion-induced hypercontracture triggers lethal reperfusion injury and its prevention results in the prevention of lethal reperfusion injury—which has been demonstrated in the BDM-treated canine hearts—lethal reperfusion injury can be avoided in the preconditioned hearts after 40 minutes of prolonged ischemia, during which the threshold level of [Ca2+]i for developing hypercontracture of the myocardium may not have been achieved.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was a standard submission to the journal. The article did not undergo external peer review.
Conflicts of Interest: The author has completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jxym-20-107). The author has no conflicts of interest to declare.
Ethical Statement: The author is accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Murry CE, Jennings RB, Reimer KA. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation 1986;74:1124-36. [Crossref] [PubMed]
- Heusch G, Gersh BJ. The pathophysiology of acute myocardial infarction and strategies of protection beyond reperfusion: a continual challenge. Eur Heart J 2017;38:774-84. [PubMed]
- Schlack W, Uebing A, Schäfer M, et al. Regional contractile blockade at the onset of reperfusion reduces infarct size in the dog heart. Pflugers Arch 1994;428:134-41. [Crossref] [PubMed]
- Koyama T, Shimada M, Baba A, et al. Effects of early reperfusion on creatine kinase release in patients with acute myocardial infarction: implications for reperfusion injury. Int J Cardiol 2012;155:335-7. [Crossref] [PubMed]
- Koyama T, Niikura H, Shibata M, et al. Possible creatine kinase washout mechanism revealed by postconditioning with lactate-enriched blood in patients experiencing ST-elevation myocardial infarctions. Int J Cardiol 2014;177:492-3. [Crossref] [PubMed]
- Koyama T. Lactated Ringer's solution for preventing myocardial reperfusion injury. Int J Cardiol Heart Vasc 2017;15:1-8. [Crossref] [PubMed]
- Dekker LR, Fiolet JW, VanBavel E, et al. Intracellular Ca2+, intercellular electrical coupling, and mechanical activity in ischemic rabbit papillary muscle. Effects of preconditioning and metabolic blockade. Circ Res 1996;79:237-46. [Crossref] [PubMed]
Cite this article as: Koyama T. An alternative viewpoint for the cardioprotective effects of ischemic preconditioning. J Xiangya Med 2021;6:10.


