
Effects of COVID-19 on the cardiovascular system and implications for management
Introduction
Coronavirus disease 2019 (COVID-2019) caused by the severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) is now a global pandemic. First reported as causing pneumonia, with acute respiratory distress syndrome (ARDS) and multiorgan dysfunction in severe cases, more data is becoming available on the extra-pulmonary manifestations of the disease. This article discusses the cardiovascular effects of COVID-19, the possible mechanisms of injury to the cardiovascular system and the implications for management.
Lessons from the MERS and SARS
Two other coronaviruses were previously known to cause severe respiratory diseases in humans, the severe acute respiratory syndrome coronavirus (SARS-CoV) and the Middle East respiratory syndrome coronavirus (MERS-CoV). The SARS-CoV-2 shares 79.6% genome identity with the SARS-CoV and both use angiotensin-converting-enzyme 2 (ACE 2) as the cell entry receptor (1).
Hypotension and tachycardia were commonly reported among hospitalized patients with severe acute respiratory syndrome (SARS) (2,3). The tachycardia happened independent of fever or hypotension and was present in some patients even on follow up 4 weeks later (2). Myocardial infarction was reported as the cause of death in some patients with SARS (4). Acute myocarditis and acute-onset heart failure has been reported in MERS patients (5). Focal myocarditis has been reported on autopsies in SARS patients (6). A prospective study looking at echocardiography in the acute phase and 30 days later in SARS patients found evidence of subclinical diastolic impairment without systolic dysfunction (7). SARS may also have long term effects on the cardiovascular system with a study suggesting altered lipid metabolism 12 years after recovering from SARS (8).
Cardivascular manifestations of COVID-19
Though most patients with COVID-19 presented with fever and/or cough (9), palpitations and/or atypical chest pain have been reported as the initial symptoms in some patients (10-12).
Acute cardiac injury defined as elevation of cardiac biomarkers (troponin-I or troponin-T) above the 99th percentile upper reference limit was prevalent in COVID-19 patients and was associated with worse outcomes. In a single center series from Wuhan, China, 416 patients among 645 consecutive cases of COVID-19 had high-sensitive troponin I measured and 82 patients (12.7%) had elevated troponin I levels (13). Lala et al. reported elevated troponin I in 985 patients (36%) among a series of 2,739 COVID-19 patients admitted to the Mount Sinai Health System in New York City for whom troponin were measured (14). In a study of 100 patients who recently recovered from COVID-19 in Germany, high sensitive troponin were detectable in 71 patients and cardiac MRI was abnormal in 78 including increased myocardial native T1 (n=73) or T2 (n=60), myocardial late gadolinium enhancement (n=32), or pericardial enhancement (n=22) (15).
Cases of fulminant myocarditis has been reported with COVID-19 (16). Though the true incidence is unknown, the limited number of reported cases suggests it is relatively rare. Findings suggestive of myocardial interstitial edema were seen on T2 short tau inversion recovery images and extensive transmural late gadolinium enhancement were noted in these cases (17). In pediatric patients, cardiac involvement including myocarditis was reported as part of the COVID-19-related multisystem inflammatory syndrome in children (MIS-C), a disease resembling Kawasaki disease (18). COVID-19-related multisystem inflammatory syndrome in adults (MIS-A) is also increasing being reported with cardiac dysfunction being a prominent feature (19). COVID-19 has also been reported as a trigger for Takotsubo cardiomyopathy (20).
EKG changes associated with ischemia, such as ST-segment elevation have been reported in COVID-19 patients (21). In a series from New York City, 18 COVID patients has been identified as having ST-segment elevation, of whom 9 patients underwent coronary angiography and 6 were found to have obstructive disease (21). Of the 18 patients, all had elevations in d-dimer levels and 13 died (21).
Arrhythmias such as atrioventricular blocks, atrial fibrillations, ventricular tachycardias and cardiac arrests have been reported in patients with COVID-19 (22). Fever has been known to increase the risk of ventricular tachycardia in patients with inherited arrhythmia syndromes (23,24). A case has been reported in which Brugada syndrome was unmasked by COVID-19 (25). Population based studies in Paris and the Lombardi region of Italy also showed an significant increase in out-of-hospital cardiac arrests coinciding with the onset of COVID-19 pandemic with significant portion of the patients having confirmed or suspected COVID-19 (26).
COVID-19 may have also long-term effects on the cardiovascular system though long-term follow-up data are not yet available. Previous studies have shown increased cardiovascular risks up to 10 years after a hospitalization for pneumonia (27). A UK study offering cardiac MRI about a month after discharge for COVID-19 patients with acute cardiac injury found 45% of the patients who does not have coronary heart disease or pulmonary embolism (PE) had myocarditis-pattern late gadolinium enhancement without evidence of edema, suggesting possible permanent scars (28).
Mechanism of cardiovascular injury
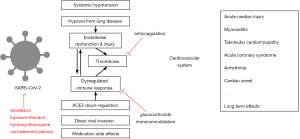
Hypoxia secondary to lung disease and systemic hypotension may cause cardiac ischemia, but several other mechanisms may be involved in cardiovascular injury.
SARS-CoV-2 may directed invade the heart. ACE 2, the cell entry receptor used by SARS-CoV-2 is widely expressed in the heart and is an essential regulator of heart function (1,29). In particular, pericytes in the heart have high expressions of ACE 2 and might act as the target cardiac cell for SARS-CoV-2 (30). ACE 2 expression in the heart is upregulated in heart diseases such as myocardial infarction and heart failure (31,32), and preexisting coronary artery disease has been identified as a strong risk factor for mortality in COVID-19 patients (33). Early results from an in vitro study suggest that SARS-CoV-2 can affect and replicate in human pluripotent stem cell-derived cardiomyocytes, impairing their electrophysiological and contractile properties (34). In one autopsy series of 39 COVID-19 patients from Germany, viral RNAs were detected in 24 patients (61.5%) (35). Other evidence, however, argues against direct viral invasion of the heart. SARS-CoV-2 cell entry depends on the serine protease TMPRSS2, which is not highly expressed in the human heart (36,37). In the German autopsy series, detection of viral RNA is not associated with an increase in inflammatory cell infiltrate into the myocardium (35). Ultimately, more data from in vitro studies and autopsies or endomyocardial biopsies are needed.
Down-regulation of angiotensin converting enzyme 2 (ACE2) may play a role in COVID-19 related organ injuries. ACE2 is an cell surface enzyme that catalyzes the conversion of angiotensin II to angiotensin-[1–7] which acts on Mas receptors counteracting the effects of the angiotensin converting enzyme (ACE)-angiotensin II (Ang II)-angiotensin type 1 receptor (AT1R) axis (38). Down-regulation of ACE2 activity in coronavirus infection may thus increase activity in the ACE-angiotensin II-AT1R axis, promoting inflammatory response, oxidative stress and fibrosis (38).
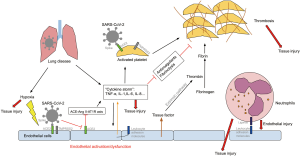
Deregulated immune response may be responsible for damage to organs including the heart in COVID-19 patients with severe disease. Serum levels of inflammatory markers such as C reactive protein (CRP) and cytokines such as IL-1β, IL-6, IL-8 and TNF-α are significantly elevated in hospitalized COVID-19 patients in a state similar to sepsis (39). This “cytokine storm” may directly cause cardiac dysfunction or cause cardiac injury through various other mechanisms including endothelial dysfunction, increased microvascular permeability, increased production of reactive oxygen species, and intravascular coagulation (40). In particular, TNF-α and IL-6 may play important roles in cytokine-induced cardiac dysfunction. TNF-α infusion caused cardiac dysfunction in animal models (41), and anti-TNF antibody can improve cardiac systolic function in patients with septic shock (42). IL-6 have been shown to cause myocardial depression through the p38 mitogen-activated protein kinase pathway in children with meningococcal sepsis (43).
Thrombosis may also represent a major mechanism for cardiovascular injury in COVID-19. Elevated D-Dimers values are common in patients with COVID-19 and thrombotic complications including PE and ischemic strokes have been reported in COVID-19 patients (10,44). Microthrombi is a prominent feature on autopsy of lungs in COVID-19 patients and extensive cardiac microthrombi without epicardial coronary artery obstruction were seen on autopsy of one COVID-19 patient who died after ST elevation myocardial infarction (45,46). Increased thrombosis in COVID-19 may be caused by direct platelet activation, activation of the coagulation cascade and inhibition of anticoagulant pathways by the inflammatory cytokines, and endothelial cell dysfunction. SARS-CoV-2 can directly activate platelets by binding of Spike protein to platelet ACE2 (47). Viral infection and cytokines such as IL-1 and TNF-α can induce expression of tissue factor by endothelial cells and mononuclear cells, activating the extrinsic pathway of coagulation (48). COVID-19 patients have been shown to have marked elevation in factor V activity, consistent with excessive activation of the coagulation pathway (49). Systemic inflammation also down-regulates physiological anticoagulant pathways, inhibits fibrinolysis, and activates platelets (50). Endothelial damage and dysfunction may play a key role in COVID-19 related organ injuries including organs in the cardiovascular system. This is accomplished by recruiting neutrophils (51), promoting thrombus formation and disrupting the regulation of vascular tone and growth. Endothelial dysfunction may be caused by direct viral infection, hypoxia, and the dysregulated immune response. Both ACE2, the cell entry receptor for SARS-CoV-2 and TMPRSS2, a serine protease helping viral entry are expressed on vascular endothelial cells (52,53). Evidence of endothelial cell infection and endotheliitis have been shown in multiple organs on autopsy series of COVID-19 patients (46). Hypoxia can activate endothelial cells, promoting its interaction with neutrophils and its procoagulant properties (54). Inflammatory cytokines such as IL-1 and TNF-α can also induce changes in endothelial cells favoring enhanced leukocyte adhesion, vasodilation, increased permeability and thrombus formation (55).
Medications used to treat COVID-19 may also adversely affect the heart. Various medications including glucocorticoids, hydroxychloroquine, azithromycin, remdesivir, tocilizumab, and traditional Chinese medicine have been used to treat patients with COVID-19. Azithromycin is known to prolong the QT interval and has been reported to cause torsade de pointes (56). Long term use of hydroxychloroquine has been associated with prolonged-QT, ventricular arrhythmias, and cardiomyopathies (57), while acute overdose may cause hypokalemia, hypotension, and ventricular tachyarrhythmias (58). Tocilizumab is associated with few cardiovascular side effects when used for rheumatoid arthritis, but it’s cardiovascular safety profile when used in this setting is less clear (59). Cases of atrial fibrillations and cardiac arrest have been reported in patients getting remdesivir though it was not clear if they were related to remdesivir (60). Certain herbal medicine has also been used in China to treat COVID-19 (61), and some of them may contain ephedra alkaloids which has been linked to hypertension and arrhythmias (62,63).
The cardiovascular effects of COVID-19 and the main mechanisms are summarized in Figure 1, while the interplay between the immune system, vascular endothelium, platelet and coagulation system in causing COVID-19 related cardiovascular injury is shown in an simplified diagram in Figure 2.
Implications for management
Acute cardiac injury as manifested by elevated troponin is an import prognostic indicator of worse outcomes and may help us with risk stratification in COVID-19 patients. In one study from Wuhan, risk stratification in COVID-19 patients with acute cardiac injury were found to have a much higher mortality (51.2% vs. 4.5%) and were more likely to require noninvasive mechanical ventilation (46.3% vs. 3.9%) and invasive mechanical ventilation (22.2% vs. 4.2%) (13). In the Mount Sinai series of COVID-19 patients for whom troponin were measured, even mildly elevated troponin I of 0.03–0.09 ng/mL were associated with death (HR 1.75, 95% CI, 1.37–2.24) and greater elevation of troponin I >0.09 ng/mL were associated with an even higher risk of death (HR 3.03, 95% CI, 2.42–3.80) (14). One study from China looking at risk factors for mortality in hospitalized COVID-19 patients report elevation in high-sensitive troponin I as the risk factor with the highest odds ratio of 80.07 (95% CI, 10.34–620.36) among other risk factors such as older age, elevated d-dimer levels, and high SOFA scores (33).
Targeting the dysregulated immune response can potentially reduce organ injury in COVID-19 and improve outcomes. Dexamethasone have been shown to reduce mortality in hospitalized COVID-19 patients requiring oxygen or mechanical ventilation (64). Though a recent trial failed to show any difference in outcomes with IL-6 receptor blocker Tocilizumab for hospitalized COVID-19 patients, trials for various other immunomodulators are still underway (65).
Given the propensity for thrombotic complications, anticoagulation may be indicated for select COVID-19 patients to prevent/treat thrombotic complications. One single center retrospective study in the US found anticoagulation to be associated with lower mortality and intubation risk among hospitalized COVID-19 patients (66) while another retrospective study in China have found an association between anticoagulation and lower mortality in severe COVID-19 patients with coagulopathy (67).
Given the inflammatory and hypercoagulable state, patients with known coronary artery disease should be managed with rigorous plaque stabilizing therapy (e.g., antiplatelet agents, statins, beta-blockers) consistent with current guidelines.
The interplay between angiotensin-converting-enzyme inhibitors (ACEIs) or angiotensin-receptor blockers (ARBs) and COVID-19 is not completely clear at this moment. While some animal studies have found increased ACE2 expression with ACEI/ARB use which could facilitate SARS-CoV-2 cell entry, other animal and human studies showed no significant change in ACE2 expression (68). Population based case-control studies in Denmark and Lombardi area of Italy failed to show any association between ACEI/ARB use and COVID-19 diagnosis (69,70) and multiple retrospective studies found no increase in mortality among COVID-19 patients who take ACEIs/ARBs (70-72). Preliminary results from the yet to be published BRACE CORONA randomized controlled trial presented at the ESC showed no difference on morality between suspending and continuing ACEIs or ARBs in hospitalized COVID-19 patients. For now, the Heart Failure Society of America, American College of Cardiology and American Heart Association have recommended those who were taking an ACEI/ARB for the right indications continue to take it.
Cardiovascular risks should be taken into consideration when prescribing medications for COVID-19 patients. Careful monitoring of QT intervals is needed when medications with potentials to prolong the QT interval are used in COVID-19 patients.
With many on-going studies, our knowledge of the effects of COVID-19 on the cardiovascular system and the underlying mechanisms will surely improve.
Acknowledgments
Funding: None.
Footnote
Peer Review File: Available at http://dx.doi.org/10.21037/jxym-20-105
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jxym-20-105). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Zhou P, Yang XL, Wang XG, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 2020;579:270-3. [Crossref] [PubMed]
- Yu CM, Wong RSM, Wu EB, et al. Cardiovascular complications of severe acute respiratory syndrome. Postgrad Med J 2006;82:140-4. [Crossref] [PubMed]
- Booth CM. Clinical Features and Short-term Outcomes of 144 Patients With SARS in the Greater Toronto Area. JAMA 2003;289:2801. [Crossref] [PubMed]
- Peiris JSM, Chu CM, Cheng VCC, et al. Clinical progression and viral load in a community outbreak of coronavirus-associated SARS pneumonia: A prospective study. Lancet 2003;361:1767-72. [Crossref] [PubMed]
- Alhogbani T. Acute myocarditis associated with novel Middle East respiratory syndrome coronavirus. Ann Saudi Med 2016;36:78-80. [Crossref] [PubMed]
- Chen J, Zhang HT, Xie YQ, et al. Zhonghua Bing Li Xue Za Zhi 2003;32:516-20. [Morphological study of severe acute respiratory syndrome (SARS)]. [PubMed]
- Li SS, Cheng C, Fu C, et al. Left ventricular performance in patients with severe acute respiratory syndrome: a 30-day echocardiographic follow-up study. Circulation 2003;108:1798-803. [Crossref] [PubMed]
- Wu Q, Zhou L, Sun X, et al. Altered Lipid Metabolism in Recovered SARS Patients Twelve Years after Infection. Sci Rep 2017;7:9110. [Crossref] [PubMed]
- Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020;395:497-506. [Crossref] [PubMed]
- Liu K, Fang YY, Deng Y, et al. Clinical characteristics of novel coronavirus cases in tertiary hospitals in Hubei Province. Chin Med J (Engl) 2020;133:1025-31. [Crossref] [PubMed]
- Zhu J, Ji P, Pang J, et al. Clinical characteristics of 3,062 COVID-19 patients: a meta-analysis. J Med Virol 2020;92:1902-14. [Crossref] [PubMed]
- Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet 2020;395:507-13. [Crossref] [PubMed]
- Shi S, Qin M, Shen B, et al. Association of Cardiac Injury With Mortality in Hospitalized Patients With COVID-19 in Wuhan, China. JAMA Cardiol 2020;5:802-10. [Crossref] [PubMed]
- Lala A, Johnson KW, Januzzi JL, et al. Prevalence and Impact of Myocardial Injury in Patients Hospitalized With COVID-19 Infection. J Am Coll Cardiol 2020;76:533-46. [Crossref] [PubMed]
- Puntmann VO, Carerj ML, Wieters I, et al. Outcomes of Cardiovascular Magnetic Resonance Imaging in Patients Recently Recovered From Coronavirus Disease 2019 (COVID-19). JAMA Cardiol 2020;5:1265-73. [Crossref] [PubMed]
- Pirzada A, Mokhtar AT, Moeller AD. COVID-19 and Myocarditis: What Do We Know So Far? CJC Open 2020;2:278-85. [Crossref] [PubMed]
- Inciardi RM, Lupi L, Zaccone G, et al. Cardiac Involvement in a Patient With Coronavirus Disease 2019 (COVID-19). JAMA Cardiol 2020;5:819-24. [Crossref] [PubMed]
- Minocha PK, Phoon CKL, Verma S, et al. Cardiac Findings in Pediatric Patients With Multisystem Inflammatory Syndrome in Children Associated With COVID-19. Clin Pediatr (Phila) 2021;60:119-26. [Crossref] [PubMed]
- Morris SB, Schwartz NG, Patel P, et al. Case Series of Multisystem Inflammatory Syndrome in Adults Associated with SARS-CoV-2 Infection — United Kingdom and United States, March-August 2020. MMWR Morb Mortal Wkly Rep 2020;69:1450-6. [Crossref] [PubMed]
- Meyer P, Degrauwe S, Van Delden C, et al. Typical takotsubo syndrome triggered by SARS-CoV-2 infection. Eur Heart J 2020;41:1860. [Crossref] [PubMed]
- Bangalore S, Sharma A, Slotwiner A, et al. ST-Segment Elevation in Patients with Covid-19 - A Case Series. N Engl J Med 2020;382:2478-80. [Crossref] [PubMed]
- Kochav SM, Coromilas E, Nalbandian A, et al. Cardiac Arrhythmias in COVID-19 Infection. Circ Arrhythm Electrophysiol 2020;13:e008719 [Crossref] [PubMed]
- Amin AS, Meregalli PG, Bardai A, et al. Fever increases the risk for cardiac arrest in the Brugada syndrome. Ann Intern Med 2008;149:216-8. [Crossref] [PubMed]
- Amin AS, Klemens CA, Verkerk AO, et al. Fever-triggered ventricular arrhythmias in Brugada syndrome and type 2 long-QT syndrome. Neth Hear J 2010;18:165-9. [Crossref] [PubMed]
- Chang D, Saleh M, Garcia-Bengo Y, et al. COVID-19 Infection Unmasking Brugada Syndrome. HeartRhythm Case Rep 2020;6:237-40. [Crossref] [PubMed]
- Baldi E, Sechi GM, Mare C, et al. Out-of-Hospital Cardiac Arrest during the Covid-19 Outbreak in Italy. N Engl J Med 2020;383:496-8. [Crossref] [PubMed]
- Corrales-Medina VF, Alvarez KN, Weissfeld LA, et al. Association between hospitalization for pneumonia and subsequent risk of cardiovascular disease. JAMA 2015;313:264-74. [Crossref] [PubMed]
- Knight DS, Kotecha T, Razvi Y, et al. COVID-19: Myocardial Injury in Survivors. Circulation 2020;142:1120-2. [Crossref] [PubMed]
- Crackower MA, Sarao R, Oliveira-dos-Santos AJ, et al. Angiotensin-converting enzyme 2 is an essential regulator of heart function. Nature 2002;417:822-8. [Crossref] [PubMed]
- Chen L, Li X, Chen M, et al. The ACE2 expression in human heart indicates new potential mechanism of heart injury among patients infected with SARS-CoV-2. Cardiovasc Res 2020;116:1097-100. [Crossref] [PubMed]
- Goulter AB, Goddard MJ, Allen JC, et al. ACE2 gene expression is up-regulated in the human failing heart. BMC Med 2004;2:19. [Crossref] [PubMed]
- Burrell LM, Risvanis J, Kubota E, et al. Myocardial infarction increases ACE2 expression in rat and humans. Eur Heart J 2005;26:369-75. [Crossref] [PubMed]
- Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 2020;395:1054-62. [Crossref] [PubMed]
- Marchiano S, Hsiang TY, Higashi T, et al. SARS-CoV-2 infects human pluripotent stem cell-derived cardiomyocytes, impairing electrical and mechanical function. bioRxiv. Available online: http://biorxiv.org/content/early/2020/08/30/2020.08.30.274464
- Lindner D, Fitzek A, Bräuninger H, et al. Association of Cardiac Infection With SARS-CoV-2 in Confirmed COVID-19 Autopsy Cases. JAMA Cardiol 2020;e203551 [Crossref] [PubMed]
- Hoffmann M, Kleine-Weber H, Schroeder S, et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell 2020;181:271-80.e8. [Crossref] [PubMed]
- Vaarala MH, Porvari KS, Kellokumpu S, et al. Expression of transmembrane serine protease TMPRSS2 in mouse and human tissues. J Pathol 2001;193:134-40. [Crossref] [PubMed]
- Ni W, Yang X, Yang D, et al. Role of angiotensin-converting enzyme 2 (ACE2) in COVID-19. Vol. 24 Crit Care 2020;24:422. [Crossref] [PubMed]
- Colantuoni A, Martini R, Caprari P, et al. COVID-19 Sepsis and Microcirculation Dysfunction. Front Physiol 2020;11:747. [Crossref] [PubMed]
- Sprague AH, Khalil RA. Inflammatory cytokines in vascular dysfunction and vascular disease. Biochem Pharmacol 2009;78:539-52. [Crossref] [PubMed]
- Eichenholz PW, Eichacker PQ, Hoffman WD, et al. Tumor necrosis factor challenges in canines: patterns of cardiovascular dysfunction. Am J Physiol 1992;263:H668-75. [PubMed]
- Vincent JL, Bakker J, Marecaux G, et al. Administration of anti-TNF antibody improves left ventricular function in septic shock patients; Results of a pilot study. Chest 1992;101:810-5. [Crossref] [PubMed]
- Pathan N, Franklin JL, Eleftherohorinou H, et al. Myocardial depressant effects of interleukin 6 in meningococcal sepsis are regulated by p38 mitogen-activated protein kinase. Crit Care Med 2011;39:1692-711. [Crossref] [PubMed]
- Klok FA, Kruip MJHA, van der Meer NJM, et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res 2020;191:145-7. [Crossref] [PubMed]
- Guagliumi G, Sonzogni A, Pescetelli I, et al. Microthrombi and ST-Segment-Elevation Myocardial Infarction in COVID-19. Circulation 2020;142:804-9. [Crossref] [PubMed]
- Ackermann M, Verleden SE, Kuehnel M, et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. N Engl J Med 2020;383:120-8. [Crossref] [PubMed]
- Zhang S, Liu Y, Wang X, et al. SARS-CoV-2 binds platelet ACE2 to enhance thrombosis in COVID-19. J Hematol Oncol 2020;13:120. [Crossref] [PubMed]
- Camerer E, Kolstø AB, Prydz H. Cell biology of tissue factor, the principal initiator of blood coagulation. Thromb Res 1996;81:1-41. [Crossref] [PubMed]
- Stefely JA, Christensen BB, Gogakos T, et al. Marked factor V activity elevation in severe COVID-19 is associated with venous thromboembolism. Am J Hematol 2020;95:1522-30. [Crossref] [PubMed]
- Levi M, Keller TT, van Gorp E, et al. Infection and inflammation and the coagulation system. Cardiovasc Res 2003;60:26-39. [Crossref] [PubMed]
- Raeburn CD, Calkins CM, Zimmerman MA, et al. Vascular cell adhesion molecule-1 expression is obligatory for endotoxin-induced myocardial neutrophil accumulation and contractile dysfunction. Surgery 2001;130:319-25. [Crossref] [PubMed]
- Bertram S, Heurich A, Lavender H, et al. Influenza and SARS-coronavirus activating proteases TMPRSS2 and HAT are expressed at multiple sites in human respiratory and gastrointestinal tracts. PLoS One 2012;7:e35876 [Crossref] [PubMed]
- Hamming I, Timens W, Bulthuis MLC, et al. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol 2004;203:631-7. [Crossref] [PubMed]
- Michiels C, Arnould T, Remacle J. Endothelial cell responses to hypoxia: initiation of a cascade of cellular interactions. Biochim Biophys Acta 2000;1497:1-10. [Crossref] [PubMed]
- Mantovani A, Bussolino F, Dejana E. Cytokine regulation of endothelial cell function. FASEB J 1992;6:2591-9. [Crossref] [PubMed]
- Huang BH, Wu CH, Hsia CP, Yin Chen C. Azithromycin-induced torsade de pointes. PACE - Pacing Clin Electrophysiol 2007;30:1579-82. [Crossref] [PubMed]
- Joyce E, Fabre A, Mahon N. Hydroxychloroquine cardiotoxicity presenting as a rapidly evolving biventricular cardiomyopathy: Key diagnostic features and literature review. Eur Heart J Acute Cardiovasc Care 2013;2:77-83. [Crossref] [PubMed]
- Marquardt K, Albertson TE. Treatment of hydroxychloroquine overdose. Am J Emerg Med 2001;19:420-4. [Crossref] [PubMed]
- Kim SC, Solomon DH, Rogers JR, et al. Cardiovascular Safety of Tocilizumab Versus Tumor Necrosis Factor Inhibitors in Patients With Rheumatoid Arthritis: A Multi-Database Cohort Study. Arthritis Rheumatol 2017;69:1154-64. [Crossref] [PubMed]
- Fan Q, Zhang B, Ma J, et al. Safety profile of the antiviral drug remdesivir: An update. Biomed Pharmacother 2020;130:110532 [Crossref] [PubMed]
- Yang Y, Islam MS, Wang J, et al. Traditional Chinese Medicine in the Treatment of Patients Infected with 2019-New Coronavirus (SARS-CoV-2): A Review and Perspective. Int J Biol Sci 2020;16:1708-17. [Crossref] [PubMed]
- Haller CA, Benowitz NL. Adverse Cardiovascular and Central Nervous System Events Associated with Dietary Supplements Containing Ephedra Alkaloids. N Engl J Med 2000;343:1833-8. [Crossref] [PubMed]
- Jia W, Wang C, Wang Y, et al. Qualitative and quantitative analysis of the major constituents in Chinese medical preparation Lianhua-Qingwen capsule by UPLC-DAD-QTOF-MS. ScientificWorldJournal 2015;2015:731765 [Crossref] [PubMed]
- RECOVERY Collaborative Group. Dexamethasone in Hospitalized Patients with Covid-19. N Engl J Med 2021;384:693-704. [Crossref] [PubMed]
- Stone JH, Frigault MJ, Serling-Boyd NJ, et al. Efficacy of Tocilizumab in Patients Hospitalized with Covid-19. N Engl J Med 2020;383:2333-44. [Crossref] [PubMed]
- Nadkarni GN, Lala A, Bagiella E, et al. Anticoagulation, Bleeding, Mortality, and Pathology in Hospitalized Patients With COVID-19. J Am Coll Cardiol 2020;76:1815-26. [Crossref] [PubMed]
- Tang N, Bai H, Chen X, et al. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemost 2020;18:1094-9. [Crossref] [PubMed]
- Sriram K, Insel PA. Risks of ACE Inhibitor and ARB Usage in COVID-19: Evaluating the Evidence. Clin Pharmacol Ther 2020;108:236-41. [Crossref] [PubMed]
- Mancia G, Rea F, Ludergnani M, et al. Renin-Angiotensin-Aldosterone System Blockers and the Risk of Covid-19. N Engl J Med 2020;382:2431-40. [Crossref] [PubMed]
- Fosbøl EL, Butt JH, Østergaard L, et al. Association of Angiotensin-Converting Enzyme Inhibitor or Angiotensin Receptor Blocker Use with COVID-19 Diagnosis and Mortality. JAMA 2020;324:168-77. [Crossref] [PubMed]
- Zhang P, Zhu L, Cai J, et al. Association of Inpatient Use of Angiotensin-Converting Enzyme Inhibitors and Angiotensin II Receptor Blockers With Mortality Among Patients With Hypertension Hospitalized With COVID-19. Circ Res 2020;126:1671-81. [Crossref] [PubMed]
- Mehra MR, Desai SS, Kuy S, et al. Cardiovascular Disease, Drug Therapy, and Mortality in Covid-19. N Engl J Med 2020;382:2582. [Crossref] [PubMed]
Cite this article as: Wu S, Zou G, Lin K, Zhang D. Effects of COVID-19 on the cardiovascular system and implications for management. J Xiangya Med 2021;6:7.


