
Role of genomics and histology diagnosis in recurrent malignant pleural effusion
Introduction
Malignant pleural effusion (MPE) is discovered based on evidence in the pleural tissue or fluid of cancerous cells and is a widespread diagnosis amongst patients with malignancy. Practically all malignant tumours can involve the pleura at an advanced stage and thus cause pleural carcinosis with pleural effusion (PE). Lung cancer is the most common cause of the MPE since the lungs’ proximity to the pleura. Secondly, the most frequent cause is metastatic breast cancer than lymphoma, ovarian, and gastrointestinal neoplasms (1). MPE is associated with a weak prognosis (median survival: 4–7 months). Even among patients whose PE are too small for treatment, survival is substantially lower than amongst patients without effusion. Survival depends primarily on tumour subtype (2).
Pathophysiology of MPE
The typical average volume of pleural fluid is about 0.26 mL/kg of body weight. A disorder to the equilibrium between absorption/production leads to PE. The inflammation reaction related to pleural carcinosis increases, as a result of increased vessel permeability, the formation of interstitial pleural fluid (1). Although PE slightly increases shunt fraction, it is unusual to discover patients with substantial hypoxemia. The associated dyspnoea is not a lung challenge due to lung collapse or to a decrease in pulmonary function. Instead, the dyspnoea is caused by the caudal displacement of the diaphragm, mechanically disadvantageous for the length-tension relationship. If the dyspnoea has been alleviated with thoracentesis, the PE was at least a significant supplier to the dyspnoea, with a reduction of the dyspnoea regardless of the lung expansion. The patient can be considered for pleurodesis if the lung has expanded, whereas a tunnelled pleural catheter is the treatment of choice if the lung is not expandable. Tunnelled pleural catheters are small bore tubes, placed in the outpatient setting, tunnelled subcutaneously into the pleural space. They permit patients or caregivers to drain PE without invasive procedures. The objectives of treating patients with MPE are:
- To enhance the quality-of-life by minimizing dyspnoea;
- To reduce thoracentesis and the demand for repetitive hospital admissions;
Early and definitive pleural palliation is recommended, given the poor prognosis of these patients (2).
Markers of MPE
More than a few new markers have been assessed with controversial results to improve the diagnostic accuracy of cytology of exudative PE. It is improbable that a single marker plays a crucial role in all types of exudative PE. The matrix metalloproteinases are a group of proteases proficient of degrading components of the extracellular matrix. The tissue-specific inhibitors of metalloproteinases prevalently control their activity. The change of the sense of balance between matrix metalloproteinases and their tissue-specific inhibitors of metalloproteinases could play a part in the pathogenesis of MPE. The matrix metalloproteinases family is constituted of at least 21 different components. Degrading Type IV collagen, matrix metalloproteinases provide to damage of integrity of the basement membrane under the mesothelial layer and around blood vessels, leading to fluid accumulation in the pleural space (3). A meta-analysis reported that a high neutrophil-to-lymphocyte ratio in the serum is correlated with overall adverse survival for several solid tumours (4). However, no study on the neutrophil-to-lymphocyte ratio of MPE has been reported. Therefore, the neutrophil-to-lymphocyte ratio in the effusion, like the neutrophil-to-lymphocyte ratio in the serum, may act as a new prognostic factor in MPE (5). The LENT score [lactate dehydrogenase in pleural fluid, Eastern Cooperative Oncology Group (ECOG) performance status, the neutrophil-lymphocyte ratio in the serum, and tumour type] could stratify patients into risk groups. It may help the guidance to therapy (Table 1) (6). Total scores of 0–1 show low risk are associated with a median survival of 319 days, as matched with a median survival of 130 days in the medium-risk category (scores 2–4) and 44 days in the high-risk group (scores 5–7). For the high-risk category, less invasive methodologies, such as positioning of a tunnelled pleural catheter, maybe most valuable, whereas low-risk patients can be treated with pleurodesis. Nonetheless, as with all medical procedures, the risks, benefits, and options should always be examined with the patient to individualize the treatment (2).
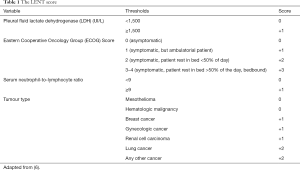
Full table
Genomics of MPE
The severity of MPE is correlated with the absence of contact between tumour-associated lymphocytes and tumour cells. A robust relationship between tumour progression and T cell functional weakening was noted and could be described by tumour’s suppressive impact on the host immune response. Regulatory T cells, a subset of the overall CD4+ lymphocytes, are well-well-defined by elevated expression of Ectonucleoside triphosphate diphosphohydrolase-1 (CD39), (5′-nucleotidase) CD73, co-stimulator for T-cell activation and survival (CD28), interleukin-2 receptor alpha chain (CD25), CTL-associated antigen-4 (CTLA-4), transcription factor FoxP3, glucocorticoid-induced tumour necrosis factor. FoxP3 seem like a relevant marker, due to its presence and upregulated expression for Regulatory T cells development and function. Regulatory T cells, expressing FoxP3, are essential for self-tolerance, keeping the balance of immunological defence, by inhibiting effector T cells. This method occurs by secretion of inhibitory cytokines, like interleukin-10 and transforming growth factor-beta or by cell-to-cell direct contact. Regulatory T cells frequencies in solid tumours and hematologic cancers were more significant than those in healthy. Regulatory T cells differentiate principally in the thymus, but this process also happens in the periphery. It is recommended that transforming growth factor-beta is involved in this process, producing differentiation of FoxP3+ Regulatory T cells from naive precursors. Thus, the capability to manage the suppressive function and/or the number of Regulatory T cells in the oncological microenvironment has a promising methodology (7).
Histological markers in MPE
The cytological diagnosis of MPE is every so often challenging, mainly because of the recurrent problem in distinguishing between tumour cells from benign reactive mesothelial cells. Other factors correlated to the cytological accuracy of low shedding of tumour cells into the pleura, histological tumour type, and the seniority of the pathologist. The immunocytochemistry achieved on slides of paraffin sections obtained from cells block of fluid samples using antibodies to differentiate amongst metastatic adenocarcinoma, reactive benign mesothelium and epithelial mesothelioma, characterize the significant difficulties faced up to pathologists and cytologists (8). Oestrogen receptors are discovered continuously in lung tissues and cell lines (mainly adenocarcinoma). Oestradiol could stimulate cancer cell migration and that an Oestrogen receptors antagonist inhibited this effect. Oestrogen promotes cell migration via Oestrogen receptors b. Osteopontin, a small integrin-binding ligand N-linked glycoprotein in controlling the cell signalling that controls tumour progression and metastasis. Enhanced osteopontin expression has been observed in the plasma of advanced cancer patients and is involved in the formation of MPE. In lung adenocarcinoma, oestradiol can augment osteopontin expression and secretion and induce cell migration through integrin binding. Although MPE had more significant vascular endothelial growth factor than those with non-MPE, there was no meaningful relationship between pleural fluid Oestradiol and vascular endothelial growth factor concentrations. Augmented pleural permeability is the principal mechanism of malignant pleural fluid development. Besides, a combination of oestrogen receptor antagonists and vascular endothelial growth factor—tyrosine kinase inhibitors reduce cell proliferation and tumour growth more than one individual treatment in both in vivo and in vitro studies. Epithelial growth factor receptor protein expression was downregulated in response to oestrogen and upregulated to anti-oestrogens in vitro. Oestrogen receptor b expression was diminished in response to epithelial growth factor and risen in response to gefitinib (9).
Management of MPE
Surgeons treating patients with MPE often rely on these progressively debated physiologic variables. Besides, they depend on their subjective assessment of the potential for prolonged survival despite pleural connection from the underlying malignancy (10). Thoracoscopic talc poudrage is the treatment of choice in these patients. The earlier it is accomplished, the better the results are, with the maintenance of the patient’s performance status as well as the quality of life. Even if pleurodesis was not a factor for better survival in MPE, pleurodesis has been implicated in prolonged survival after pleurodesis. Pleurodesis might act directly on cancer cells by inducing apoptosis, or it might inhibit neoangiogenesis (10). The association of complementary techniques for pleural fluid cytology enhances diagnostic accuracy and consents the usage of thoracentesis samples. The most employed methods include analysis of DNA ploidy by flow or static cytometry and immunocytochemical techniques. The assessment of DNA ploidy is laborious and not adds significant sensitivity to the cytology. Currently, fluorescence in situ hybridization (FISH) is utilized in the cancer diagnosis since it identifies cells with gene changes and structural and/or numerical chromosome abnormalities (8).
Acknowledgments
Funding: This work was partially supported by the Italian Ministry of Health with Ricerca Corrente and 5×1000 funds.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Duilio Divisi, Roberto Crisci) for the series “Malignant Pleural Effusion” published in Journal of Xiangya Medicine. The article has undergone external peer review.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jxym-2019-mpe-02). The series “Malignant Pleural Effusion” was commissioned by the editorial office without any funding or sponsorship. LB serves as an unpaid editorial board member of Journal of Xiangya Medicine from Aug 2019 to Jul 2021. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ried M, Hofmann HS. The treatment of pleural carcinosis with malignant pleural effusion. Dtsch Arztebl Int 2013;110:313-8. [Crossref] [PubMed]
- Feller-Kopman D, Light R. Pleural Disease. N Engl J Med 2018;378:740-51. [Crossref] [PubMed]
- Fiorelli A, Ricci S, Feola A, et al. Matrix metalloproteinase-9 and tissue inhibitor of metalloproteinase-1 in diagnosis of pleural effusion of malignant origin. Interact Cardiovasc Thorac Surg 2016;22:411-8. [Crossref] [PubMed]
- Templeton AJ, McNamara MG, Šeruga B, et al. Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: a systematic review and meta-analysis. J Natl Cancer Inst 2014;106:dju124 [Crossref] [PubMed]
- Lee YS, Nam HS, Lim JH, et al. Prognostic impact of a new score using neutrophil-to-lymphocyte ratios in the serum and malignant pleural effusion in lung cancer patients. BMC Cancer 2017;17:557. [Crossref] [PubMed]
- Clive AO, Kahan BC, Hooper CE, et al. Predicting survival in malignant pleural effusion: development and validation of the LENT prognostic score. Thorax 2014;69:1098-104. [Crossref] [PubMed]
- Budna J, Kaczmarek M, Kolecka-Bednarczyk A, et al. Enhanced Suppressive Activity of Regulatory T Cells in the Microenvironment of Malignant Pleural Effusions. J Immunol Res 2018;2018:9876014 [Crossref] [PubMed]
- Rosolen DC, Kulikowski LD, Bottura G, et al. Efficacy of two fluorescence in situ hybridization (FISH) probes for diagnosing malignant pleural effusions. Lung Cancer 2013;80:284-8. [Crossref] [PubMed]
- Hsu LH, Liu KJ, Tsai MF, et al. Estrogen adversely affects the prognosis of patients with lung adenocarcinoma. Cancer Sci 2015;106:51-9. [Crossref] [PubMed]
- Anevlavis S, Kouliatsis G, Sotiriou I, et al. Prognostic factors in patients presenting with pleural effusion revealing malignancy. Respiration 2014;87:311-6. [Crossref] [PubMed]
Cite this article as: Bertolaccini L, Spaggiari L. Role of genomics and histology diagnosis in recurrent malignant pleural effusion. J Xiangya Med 2021;6:5.


