
Acute coronary syndrome with rare coincidence of bilateral coronary artery-pulmonary artery fistulas a case report
Introduction
Coronary artery anomalies are relatively rare in the general population with prevalence about 1% (1). Coronary artery fistula (CAF) is a type of coronary artery anomaly in which an abnormal vessel originating from a coronary artery bypasses the normal capillary bed and terminates within the great vessels or cardiac chambers. The prevalence of CAFs on CT coronary angiogram (CAG) is 0.9% (2-4). Bilateral coronary pulmonary artery fistulas is an extremely rare occurrence. We report a case of acute coronary syndrome with bilateral coronary pulmonary artery fistulas.
We present the following case in accordance with the CARE reporting checklist (available at http://dx.doi.org/10.21037/jxym-20-82).
Case presentation
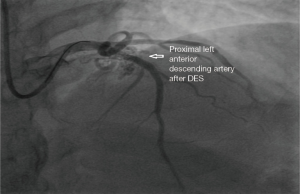
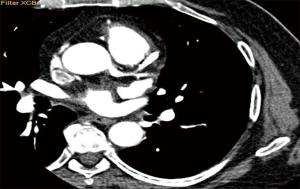
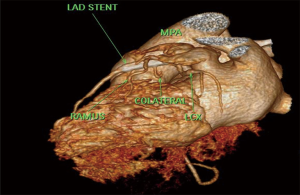
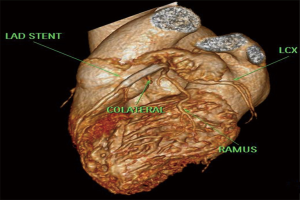
A 65 years diabetic male patient with no other comorbidities and no significant past and family history presented with anterior wall ST elevation myocardial infarction (STEMI). Cardiac examination was clinically normal. Echocardiogram showed regional wall motion abnormalities in the anterior wall with ejection fraction of 48%, no chamber dilatation and normal pulmonary arterial pressure. Chest X-ray was normal. Patient was treated with fibrinolytic agent. Subsequently CAG was done revealed, left main (LM)—normal, left anterior descending (LAD) artery—proximal 90% stenosis, left circumflex (LCX) artery—normal, right coronary artery (RCA)—normal. Incidentally CAG revealed coronary pulmonary arterial fistula (CPAF) with small multiple leash of vessels originating from LM, proximal LAD and RCA had fistulous communication to main pulmonary artery (Figures 1-4, Videos 1-4). Percutaneous coronary intervention (PCI) was performed to LAD with drug eluting stent (DES) (Figure 5, Video 5) and no intervention for CPAF in view of small and multiple vessels which were hemodynamically insignificant (normal pulmonary artery pressure and Qp/Qs:1.2). Patient was discharged with uneventful recovery. CT CAG was done also revealed bilateral coronary to pulmonary artery fistula (Figures 6-8). On follow-up after 4 months patient remained symptom free and Ecg, Echo were normal. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient.
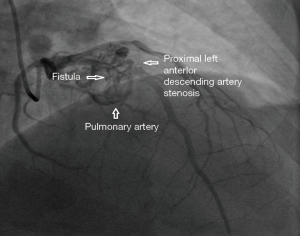
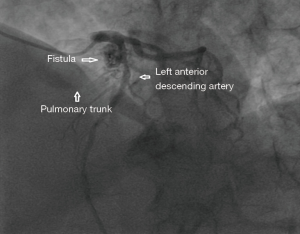
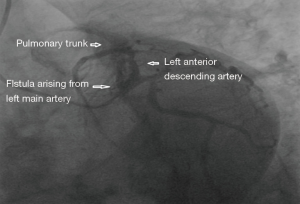
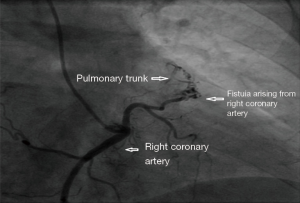
Discussion
CAFs are usually congenital or rarely acquired connections between the coronary vessels and cardiac chambers or other vascular structures such as vena cava, pulmonary artery or pulmonary veins. The incidence of CAF is 0.1% to 0.2% in all patients undergoing CAG. These fistulas may arise from any of the major coronary arteries however in most cases CAF arises from the RCA. CAFs may affect hemodynamic parameters (3) and can cause various complications including arrhythmias, myocardial ischemia, infective endocarditis and heart failure in adults (5). CAFs were first reported in 1865 (6) by Krause, followed by Haller and Little (7), author described clinical triad of a CAF: a cardiac murmur, a large tortuous coronary artery and atrial or ventricular left-to-right shunt. The incidence of CPAFs has increased as cardiac CT angiography has become widely used, with these fistulas constituting 15–30% of all CAFs (4,8). The most accepted embryologic explanation for a CPAF is the Hackensellner involution-persistence hypothesis. According to this theory among the six branches of the truncus, only two branches from the aortic sinus remain to form the coronary arteries, while rest of the branches involutes. A CPAF is formed due to abnormal persistence of branch of pulmonary sinus and connects to the normal branches from the aortic sinus coronary arteries, pulmonary sinus normally involutes during embryonic life (4). At cardiac CT angiogram CPAF shows contrast shunt sign appearance of an abnormal contrast blush, in a relatively less opacified pulmonary trunk or as a well visualized fistulous tract between the pulmonary trunk and coronary artery (9). According to a study by Verdini et al. (9), two types of CPAF occur. The first type is multiple small calibre fistulous connections from the RCA or LAD, which connects to the main pulmonary trunk. Second type is a single prominent fistulous connection between the LAD or RCA and the main pulmonary trunk. A CPAF with multiple connections is less likely to have hemodynamic disturbance related symptoms than single prominent fistulous connection. According to Kim et al. (4), CT angiography shows 59% of CAFs have multiple pulmonary artery origins. This is higher than other studies with conventional coronary angiography which reported 11–16%. The difference may be due to the higher spatial resolution of CT (4). The Vieussens ring and the collateral pathway between the conus branches of the RCA and LAD is often seen along the pre pulmonary trunk in cases of multiple arterial origins in patients with coronary to pulmonary fistulas. In a study by Verdini et al. (9), a total of 103 CPAF patients were evaluated ages ranging from newborn to 88 years. According to this study multiple coronary arteries are rarely involved. Most common involved artery is RCA. This series demonstrates that multiple fistulas are commonly present, with the most common pattern being between the LM/LAD and the main pulmonary trunk. Bilateral fistulas originating from both the coronary systems account for 5% of the total. These types of fistulas terminate more often into the pulmonary artery (56%) than the unilateral fistulas (17%) (10).
In our patient who presented with acute coronary syndrome STEMI with proximal LAD stenosis, initially fibrinolysis followed by PCI (pharmacoinvasive) was done. Incidentally bilateral CAF to pulmonary artery was noticed during PCI and was treated conservatively. On follow-up after 4 months patient remained symptom free. Hemodynamically significant fistula may present with angina due to coronary steal, cardiac failure, arrhythmias. If the patient has hemodynamically significant CPAF following PCI on follow-up if he presents with angina then either instent restenosis or coronary steal phenomenon should be considered. We are reporting a rare case of bilateral CAF to main pulmonary artery with acute coronary syndrome.
Conclusions
We are presenting a case of bilateral CPAF with STEMI which was treated with pharmacoinvasive therapy and conservative management for CPAF. CAF is thought to be present in 0.002% in general population. Multiple fistulas such as in our case being much rarer occurring in 10% to 16% of all CAFs, with involvement of both coronaries is less than 5%. Bilateral coronary pulmonary artery fistula with acute coronary syndrome is very rarely reported in the literature to the best of our knowledge. The main indication for closure of CAF are patient symptoms, heart failure, coronary ischemia, significant shunting. Treatment options include observation, only for hemodynamically significant and symptomatic patients transcatheter embolization or surgical intervention.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at http://dx.doi.org/10.21037/jxym-20-82
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jxym-20-82). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Zenooz NA, Habibi R, Mammen L, et al. Coronary artery fistulas: CT findings. Radiographics 2009;29:781-9. [Crossref] [PubMed]
- Vavuranakis M, Bush CA, Boudoulas H. Coronary artery fistulas in adults: incidence, angiographic characteristics, natural history. Cathet Cardiovasc Diagn 1995;35:116-20. [Crossref] [PubMed]
- Yamanaka O, Hobbs RE. Coronary artery anomalies in 126,595 patients undergoing coronary arteriography. Cathet Cardiovasc Diagn 1990;21:28-40. [Crossref] [PubMed]
- Kim MS, Jung JI, Chun HJ. Coronary to pulmonary artery fistula: morphologic features at multidetector CT. Int J Cardiovasc Imaging 2010;26:273-80. [Crossref] [PubMed]
- Vitarelli A, De Curtis G, Conde Y, et al. Assessment of congenital coronary artery fistulas by transesophageal color Doppler echocardiography. Am J Med 2002;113:127-33. [Crossref] [PubMed]
- Krause W. Ueber den Ursprung einer accessorischen A. coronaria cordis aus der A. pulmonalis. Z Rat Med 1865;24:225-9.
- Haller JA Jr, Little JA. Diagnosis and surgical correction of congenital coronary artery-coronary sinus fistula. Circulation 1963;27:939-42. [Crossref] [PubMed]
- Tachibana M, Mukouhara N, Hirami R, et al. Double congenital fistulae with aneurysm diagnosed by combining imaging modalities. Acta Med Okayama 2013;67:305-9. [PubMed]
- Verdini D, Vargas D, Kuo A, et al. Coronary-pulmonary artery fistulas: a systematic review. J Thorac Imaging 2016;31:380-90. [Crossref] [PubMed]
- Baim DS, Kline H, Silverman JF. Bilateral coronary artery--pulmonary artery fistulas. Report of five cases and review of the literature. Circulation 1982;65:810-5. [Crossref] [PubMed]
Cite this article as: Ravindranath KS, Rehaman A, Singh H, Karur S. Acute coronary syndrome with rare coincidence of bilateral coronary artery-pulmonary artery fistulas a case report. J Xiangya Med 2020;5:42.


