
Biomarkers in the diagnosis and treatment of malignant pleural disease: an integrative review
Introduction
Mesothelioma is an aggressive tumor arising from the mesothelial surface. Mesothelioma occurs in the pleural cavity (PM) and peritoneum in approximately 90% and 10% of cases, respectively. Primary paratesticular and pericardial mesothelioma are very rare (1-3). It is considered a relatively rare neoplasia, accounting of 0.2% of all tumors, with an incidence of 3.4/100,000 patients. Occupational exposure to asbestos is considered the main risk factor in adult patients, since more than 90% of the cases have a history of asbestos exposure. In the last 10 years, the incidence of PM has increased slightly, mainly due to the 30–50 years delay between exposure to asbestos and development of malignant cell growth. Asbestos fibers, alone or with other risk factors, such as a particular genetic background and/or viral infections can cause genetic alterations responsible for the transformation of normal mesothelial cells (4-6). In most cases, mesothelioma is a sporadic neoplasm, but familial neoplastic forms, characterized by germline mutations of the BAP1 gene have been described. In addition to mesothelioma, germline BAP1 mutations confer increased susceptibility for the development of several other tumors including uveal melanoma, cutaneous melanoma, and renal cell cancers (7,8).
Patients typically experience weight loss, pain and shortness of breath. Unilateral serum effusions are also often present on clinical examination. These signs and symptoms can manifest themselves for many months. Diagnosis is based on clinical and radiological characteristics, although it is necessary to have a detailed professional history. Samples for diagnosis can be obtained from pleural effusions by thoracentesis.
However, tissue samples obtained by ultrasound-guided biopsies, video-assisted thoracic surgery (VATS) or debulking samples should be examined whenever clinically possible (4-6,9).
Prognosis is poor due to the limited therapeutic options and difficulty in complete surgical removal. According to Global Cancer Observatory (World Health Organization) data [2018], 25,576 patients died for PM. The patients not treated have a median survival time of 6 months and most patients die within 24 months of diagnosis (10).
However, the progress of the last years in the knowledge of genetic and immunophenotype characteristics of mesothelioma could be useful for innovative pharmacological treatments in the next future. With this aim, we made a selection of the most reliable and recent data emerging from the literature, by searching original articles published in indexed journals in MEDLINE in the period between 1992 and 2020, to propose an updated state of the art. Several other sources have also been considered, including some most-viewed Web sites (http://www.pathologyoutlines.com/; http://med.stanford.edu/pathology.html; www.clinicaltrials.gov) and the latest guidelines [College of American Pathologists (CAP) guidelines 2017; American Society of Clinical Oncology (ASCO) 2018; ERS/ESTS/EACTS/ESTRO guidelines 2020]. We present the following article in accordance with the Narrative Review reporting checklist (available at http://dx.doi.org/10.21037/jxym-20-79).
Pathological diagnosis and prognosis
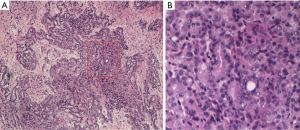
Pathological diagnosis of PM, according to the 2015 classification of the World Health Organization, can sometimes be very complicated, due to the heterogeneity of neoplastic cells and the propensity of mesothelioma cells to mimic other forms of cancer. For this reason, it generally requires a multidisciplinary approach through a correlation of cytological and histological characteristics with clinical and radiological data. The “epithelioid” is the main histotype, accounting for 60–75% of all cases. It is the variant with the largest number of cytoarchitectural patterns often mixed together (trabecular, microcystic, papillary, tubulo-papillary, micropapillary, solid, with clear cells, ring with bezel or small cells) which mimic an epithelial neoplasm, a lymphoma or a lymphoepithelial-like carcinoma (Figure 1). Often it causes pleural serum effusions and is related to a good prognosis (12–27 months after diagnosis) (11). Sarcomatoid histotype accounts for 10–15% of all cases of mesotheliomas. It is characterized by the presence of a proliferation of spindle cells arranged in short bundles with a history form or disordered pattern that infiltrates the parietal pleura or the pulmonary parenchyma. The nuclear atypia and mitosis can be of varying degrees, from absent to prominent (Figure 2). The presence of tumor necrosis is relevant for the diagnosis. A variant of sarcomatoid PM is mentioned as desmoplastic if >50% of the tumor shows desmoplastic pattern. The desmoplastic variant is usually the most difficult form to diagnose. It is a mild proliferation of spindle elements arranged in a disordered manner, in a hyalinized collagen stroma. Invasion of soft tissue or lung parenchyma is sometimes difficult to demonstrate, as immunohistochemical testing for the mesothelium are often negative. Sarcomatoid phenotype and its desmoplastic variant are related to an unfavorable prognosis, with an overall survival of 4-18 months after diagnosis (11).
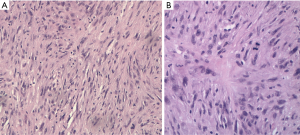
The Biphasic form of PM, 10–25% of all cases, is a combination of the epithelioid and sarcomatoid pattern: each component should represent at least 10% of the tumor for the diagnosis of this variant and it is recommended to indicate the percentages of the respective components in the report (11). The overall survival of biphasic subtype varies from 8 to 21 months.
Often, the first biological material available for pathological diagnosis is the cytological sample, particularly for patients with epithelioid mesothelioma which have a serous pleural effusion in up to 90% of cases. Sarcomatoid and biphasic mesotheliomas rarely cause large pleural effusions and, when this occurs, these subtypes do not present a large exfoliate neoplastic cellular component, the neoplastic cells are rare and difficult to evaluate (12,13). Diagnosis on cytological material is reliable but requires the presence of both cytological characteristics of malignancy and evidence of the expression of mesothelial biomarkers (14).
The first clinical question for the pathologist is the origin of the atypical cells and, once the mesothelial origin has been ascertained, when necessary, it is essential to distinguish between benign pleural serous effusions, which can present mesothelial reactive cells, and malignant pleural effusions. The presence of tumor cells in pleural effusions may also depend on a metastatic localization of a known primary tumor at another site, but also when patients are affected by other known malignancies, the possibility of a coexisting mesothelial neoplasms must be taken into consideration (12-15). It should also be noted that three-dimensional aggregates of atypical mesothelial cells may be present in benign effusions related to heart failure, cirrhosis or benign ovarian pathology (12).
However, cytopathological diagnosis should be confirmed on tissue obtained by guided ultrasound biopsies, VATS or debulking surgery. Indeed, the evaluation of vascular or stromal invasions is necessary for a differential diagnosis with the pleurisy. To distinguish mesothelioma from pleurisy, a full-thickness biopsy is required in order to visualize neoplastic growth from the pleural surface towards the underlying areas because only in this way it is possible to recognize the stromal invasion (12,16-18). On the other hand, invasion can be difficult to recognize when tissue sample is small. In this case, the presence of infiltrating neoplastic cells may not be evident due to the difficulties related to the orientation of small biopsy in paraffin block. In case of proliferation of atypical mesothelial cells, early invasive PM should be suspected and if there is no clear sign of stromal invasion, the diagnosis of “atypical mesothelial proliferation” should be made and further sampling should be suggested (16). On the contrary, cell growth sometimes mimics neoplastic infiltration. This is the case of the “fake fat” in the pleuritis, a very common diagnostic pitfall due to a traction artifact caused by biopsy procedure. “Fake fat” is a pattern characterized by round or elongated spaces in fibrotic tissue, parallel to the pleural surface with mesothelial cells in the middle (19).
For the diagnosis of mesothelioma major and minor criteria are required. The main criteria are hypercellularity, solid or diffuse cell growth and a zonation. The so-called zonation is referred to a pattern characterized by hypercellularity at the surface while a reduced cellularity is observed in deep. Cellular atypia, mitoses and the presence of necrosis are considered to be minor criteria because they are also present in mesothelial reactive hyperplasia. However, severe atypia and numerous atypical mitotic figures are in favor of PM (17-20).
Old and new biomarkers for the diagnosis and prognosis of mesothelioma
The diagnosis of PM must be based both on morphology and on an appropriate immunophenotypic characterization of neoplastic cells. This allows to carry out a differential histological diagnosis of PM versus primary lung cancer or epithelioid/biphasic mesothelioma versus reactive mesothelial proliferations and sarcomatoid PM versus fibrinoid pleuritis. Several specific biomarkers for mesothelial and epithelial cells can be searched on large tissue resections, small biopsies and cell blocks. Therefore, an adequate amount of tissue or cells is a crucial parameter in order to be able to test all needed biomarkers, and a small biopsy may not be representative of the tumor (12,21).
Malignant mesothelioma vs. malignant epithelial cell proliferations
The differential diagnosis between mesothelioma and malignant epithelial neoplasia is sometimes difficult. For this reason, analysis of multiple biomarkers can allow a more accurate diagnosis. In some particular cases, it is advisable to proceed with a multi-step algorithm, based on the morphological aspect of the neoplasm (22,23). The first biomarkers to be used are those dedicated to the identification of the mesothelial cell line. Broadly speaking, mesothelial neoplastic cells have been reported to overexpress some proteins and in particular calretinin, Wilms tumor 1 (WT1) and-cytokeratins 5 and 6 (Ck5/6) (Figure 3). Calretinin exhibits immunoreactivity in the cytoplasm and nucleus of mesothelial cells, while WT1 protein shows only nuclear expression and the immunoreactivity for CK5 and CK6 is evident only in the cytoplasm. These characteristics must be considered to recognize tissue artifacts or methodological defects (24-27). However, the loss of expression of the aforementioned proteins, does not exclude the diagnosis of PM because about 30% of mesothelial malignancies have a “null phenotype” (21). Other markers that are useful for establishing the mesothelial cell line include epithelial membrane antigen (EMA), podoplanin (D2-40), thrombomodulin and more recently HEG1 (28-30). In recent studies, HEG1 has proven to be the most sensitive and specific marker in the different variants of mesothelioma and in the differential diagnosis with pulmonary adenocarcinoma. EMA is a mucin-like transmembrane glycoprotein with membrane immunoreactivity. Thrombomodulin (CD141) is an endothelial transmembrane glycoprotein localized on the surface of endothelial cells, while mesothelin is a glycoprotein of the surface of normal mesothelial cells. Since 2005, several studies reported, in mesothelial neoplastic cells, the expression of podoplanin, a transmembrane mucoprotein recognized by the D2–40 monoclonal antibody (31-33). Specificity and sensitivity for these biomarkers are summarized in Table 1. Unfortunately, some markers are not so specific for mesothelial cells. Calretinin and CK5/6 well recognize breast and squamous cell carcinomas (34). Mesothelin protein can be expressed in pancreatic adenocarcinoma.
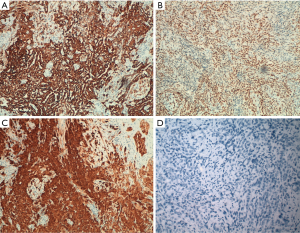
Table 1
| Mesothelioma marker | Sensitivity | Specificity |
|---|---|---|
| Calretinin | >90% | 90–95% |
| CK5/6 | 75–100% | 80–90% |
| D2–40 | 90–100% | 85% |
| WT1 | 70–95% | 100% |
| Mesothelin | 90% | 60–70% |
| Thrombomodulin | 74% | 90–95% |
| EMA | 85% | 1% |
EMA, epithelial membrane antigen; WT1, Wilms tumor 1.
In clinical practice, when in doubt, it is mandatory to consider other primary sites and distinguish mesothelioma from lung carcinoma or pleural metastasis from other sites (kidney, breast, ovary etc.) (Table 2). To rule out the possibility of epithelial neoplasia, a variety of biomarkers such as CEA, MOC-31, B72.3 and BER-EP4 must be tested (35). In particular, to exclude lung primitivity the most common biomarkers are TTF1 and Napsin A, which show immunoreactivity in lung adenocarcinoma, and p40 expressed in squamous cell carcinoma (35-38) (Figure 3). Based on MESOPATH studies, TTF1 and calretinin biomarkers allow to differentiate lung adenocarcinoma from epithelioid mesothelioma which is positive for calretinin and negative for TTF1 (39-43). In differential diagnosis with breast cancer, the expression of estrogen (ER) and progesterone receptors (PR) is useful, considering that over 75% of breast carcinomas show immunoreactivity for ER/PR.
Table 2
| Biomarkers | Expression in mesothelioma (%) | Malignancies with positive immunoreactivity |
|---|---|---|
| Calretinin (nuclear and cytoplasmic) | 55–90 | Sex cord stromal tumors (50–100%), giant cell carcinoma (67%), large cell (38%), small cell (49%) |
| CAM 5.2 | 98–99 | Carcinomas (90–100%) |
| WT1 (nuclear) | 64–82 | Carcinomas of the gynecologic tract (e.g., serous carcinomas) (80%), Wilms tumor (100%) |
| CK5, CK5/6 | 28–93 | Squamous cell carcinoma (77%), NUT carcinoma (87%), breast carcinoma (97%), urothelial carcinoma (63%) |
| Keratin AE1/AE3 | 84–100 | Carcinomas (98–100%) |
| Podoplanin (D2–40) | 43–80 | Lung squamous cell carcinoma (50%), follicular dendritic cell tumor (100%), angiosarcoma (94%), seminoma (100%), serous carcinoma (23%), lung adenocarcinoma (<15%) |
| GATA3 (nuclear) | 32 | Breast carcinoma (72–94%), urothelial carcinoma (67–93%) |
| BerEP4 | 0–14 | Adenocarcinoma (10–90%), squamous cell carcinoma (85–100%) |
| MOC31 | 0–14 | Lung adenocarcinoma (95–100%), most carcinomas (71–90%) |
| B72.3 | 4–13 | Adenocarcinoma (90–100%), squamous cell carcinoma (75–85%) |
| pCEA | 1–5 | Lung adenocarcinoma (80–100%), squamous cell carcinoma (80%), neuroendocrine tumors (35–70%) |
| MUC4 | 0 | Adenocarcinoma (89–100%), squamous cell carcinoma (83%) |
| Claudin 4 | 0 | Lung adenocarcinoma (99–100%), lung squamous cell carcinoma (78–92%), sarcomatoid carcinoma (33%) |
| TTF1 | 0 | Lung adenocarcinoma (75–85%), thyroid carcinoma (90–100%) |
| p40 | 2 | Lung squamous cell carcinoma (100%), thymic carcinoma (50–100%), NUT carcinoma (67%), urothelial carcinoma (90–95%) |
| NAPSIN-A | 0 | Lung adenocarcinoma (80%), renal cell carcinoma (83% papillari and 33% clear cell) |
| CDX2 | 0 | Pancreatobiliary adenocarcinoma (22–36%), small intestinal adenocarcinoma (60%), colorectal adenocarcinoma (86–100%) |
| Estrogen receptor | 0 | Breast carcinoma (depending on subtypes: 80–90%), carcinomas of gynecologic tract (70–90%) |
| PAX8 | 3–13 | Renal cell carcinoma (90%), thyroid carcinoma (90%), carcinoma of gynecologic tract (90–96%) |
Some biomarkers, such as CD15, PAX8 and PAX2, allow to identify an epithelial kidney cancer (44). Gastrointestinal tract adenocarcinomas can be recognized for the CDX2 expression, while prostate carcinoma for the prostate- specific antigen (PSA) (12,21,45).
Recently, the International Mesothelioma Interest Group reported recommendations on relevant biomarkers and suggested to use at least 2 positive biomarkers in neoplastic mesothelial cells such as calretinin, WT1 protein, keratin 5/6 or podoplanin and 2 positive biomarkers in epithelial malignancies among TTF1, MOC-31, B72.3, CEA, BER-EP4, CD115 and ER-a (see below). Therefore, no specific panel is proposed, but the International Mesothelioma Group recommends that each laboratory should choose antibodies with a sensitivity and specificity of at least 80% for immunohistochemical evaluation (18). In addition, the selected markers should be supplemented with other biomarkers for addressing the differential diagnosis in particular situations (Table 2).
Epithelioid mesothelioma vs. reactive mesothelial proliferation
Reactive mesothelial proliferation can be difficult to distinguish from mesothelioma because it often shows necrosis, cytological atypia, high proliferation rate, high cellularity and entrapment of mesothelial cells within fibrosis with histological aspects that can mimic neoplastic invasion. The real invasion can be demonstrated by the expression of pan-cytokeratin and in epithelioid mesothelioma also by calretinin expression. Indeed, as described above, a very common diagnostic pitfall is the “fake fat” due to a traction artifact. This morphological aspect mimics neoplastic invasion and in difficult cases, the EMA expression with an accentuated reactivity at the cell membrane level and the absence of desmin immunoreactivity have been reported as useful biomarkers for mesothelioma diagnosis. International studies recommended the combined use of desmin/EMA staining to overcome difficulties (46).
In addition, recent studies suggested that BRCA-associated protein-1 (BAP1) and ciclin-dependent kinase inhibitor 2 (CDKN 2, p16) are very useful in differentiating benign from malignant mesothelial proliferation. The absence of BAP1 expression has been associated with malignant mesothelial proliferations and represents the most reliable and specific diagnostic marker (when negative) even on cytological samples, since all reactive mesothelial processes are positive for the expression of BAP1 (47).
The BAP1 gene is located on the chromosome band 3p21, and encodes BAP1 protein that is a deubiquitinase, involved in the removal of ubiquitin from H3a (mono-ubiquitin) and from targets as BRCA1, BARD1 and HCF1, a transcription co-factor. BAP1 is involved in cellular growth, regulation of DNA transcription and cell cycle, response to DNA damage and chromatin remodeling (48).
Indeed, germline BAP1 mutations have been associated with a familial syndrome characterized by increased risks to develop different cancer types as skin basal cell carcinoma, uveal and cutaneous melanoma, breast and renal cell carcinoma, sarcomas, brain tumors and malignant mesothelioma. Somatic mutations in the BAP1 gene occur in up to 50% of sporadic mesotheliomas (7,8,48). The most common gene alterations are point mutations and bi-allelic inactivating deletions that cause the truncated protein. The result is a degradation of the BAP1 protein with loss of expression, easily documented by loss of immunoreactivity which shows a high concordance with the presence of the BAP1 mutation. A subset of cases can only have cytoplasmic staining resulting from the loss of the BAP1 autodeubiquitination function and this type of altered expression should also be considered positive. Loss of BAP1 expression is reported in 60% of epithelioid and biphasic mesothelioma and in 20% of sarcomatoid type, Therefore, in clinical practice, BAP1 is an important biomarker as it allows to distinguish, by immunostaining of tumor sections, between malignant (loss of BAP1expression) and benign (presence of expression) mesothelial proliferations (49-55).
The homozygous deletion of the CDKN2A gene is reported in half of the cases of mesothelioma. P16 plays a very important role in the regulation of the cell cycle and is recently described in aging. Therefore, the high mutation frequency could be caused by oxidative stress and inflammatory background, both induced by exposure to asbestos. The analysis of CDKN2a can be performed by fluorescence in situ hybridization (FISH) and a homozygous deletion is reported in up to 70% of primary epithelioid and 90% to 100% of sarcomatoid PM. It is important to highlight that benign mesothelial reactive proliferation is not characterized by CDKN2A deletion with a specificity of 100%.
Some studies have revealed frequent co-deletion of the MTAP gene (which encodes methylthioadenosine phosphorylase) and CDKN2A at the 9p21 locus. The loss of MTAP protein expression has been described as a surrogate for CDKN2A deletion and is relatively easy to diagnose, considering the excellent inter-observer reproducibility (47,56-58). Loss of cytoplasmic MTAP immunostaining shows a sensitivity of 65–88% and a specificity of 96–100% for CDKN2A homozygous deletion. In addition, BAP1 and MTAP expression are documented in PM but not in mesothelial reactive proliferations with a specificity of 100% and sensitivity of 60% and 40%, respectively. If BAP1 and MTAP are evaluated simultaneously, the sensitivity is higher (77%). Indeed, the recent literature does not report MTAP loss in benign mesothelial lesions (59,60).
Recently, 5-hmC is described for mesothelioma diagnosis. It is a modified nucleotide, produced from 5-methylcytosine by the TET family of DNA hydroxylases, that is the first step for DNA demethylation. Decreased levels of 5-hmC with loss of staining in greater than 50% of tumor cells have demonstrated high sensitive (92%) and 100% of specificity for malignancies. Until now, there are few studies published and a larger multi-institutional study will be necessary to confirm these data (60,61).
Finally, enhancer of zeste homolog 2 (EZH2) that is a component of polycomb repressive complex 2 (PRC2) has a central role in epigenetic suppression of gene expression through trimethylation of a critical lysine residue in histone 3 (H3K27). EZH2 overexpression is reported in a range of malignancies, and a link between BAP1 loss and overexpression of EZH2 has been described. The first work showed significantly higher EZH2 expression levels in malignant mesothelioma compared to reactive mesothelial pleuritis. Today EZH2 is considered a promising marker of malignant mesothelioma, however it needs validation in larger multi-institutional studies (62,63).
Sarcomatoid mesothelioma versus fibrous pleuritis
When there is a proliferation of spindle cells, the stromal invasion can sometimes be difficult to recognize, but reactive fibrous pleurisy can be highlighted with pancytokeratin staining. It is important to note that CK7 is more expressed in sarcomatoid mesothelioma compared to reactive spindle cell proliferations. In these cases, it is really important to identify where pancytokeratin positive cells are localized. If the immunoreactive cells are present in adipose tissue or skeletal muscle tissue or lung tissue, it is possible to formulate a diagnosis of mesothelioma (64).
In some cases of organizing pleuritis, cytokeratin positive cells, horizontally oriented, may be present around the fat like spaces. Additional biomarkers such as S100 protein, laminin and collagen IV may help to differentiate sarcomatoid mesothelioma from “fake fat” which shows no expression of these biomarkers (12,21,65).
Sarcomatoid mesothelioma versus other malignancies
The heaviest diagnostic difficulties are encountered with sarcomatoid/desmoplastic mesothelioma forms because most sarcomatoid mesotheliomas tend to lose the expression of the classic mesothelial markers and maintain only the positivity for cytokeratins (CAM5.2 low molecular weight cytokeratin cocktail or other cocktails such as AE1/AE3 and MNF116). For this reason, these biomarkers have been included in CAP guidelines. The positive immunoreactivity for cytokeratins can be particular useful for detecting neoplastic cells and infiltration of these cells into soft tissues. The other biomarkers previously described in epithelioid mesothelioma as CK5/6, WT1, claudin 4, CEA, Ber-EP4 and MOC31 are not useful because they are completely negative in the sarcomatoid subtype. Sarcomatoid mesothelioma can exhibit calretinin expression in around 30% of the cases and podoplanin in a variable percentage of the cases (21).
The differential diagnosis with other malignant tumors includes mainly the sarcomatoid carcinoma of lung and kidney, synovial sarcoma, angiosarcoma and melanoma. For sarcomatoid lung carcinoma, a panel of biomarkers including TTF1, napsin-A and p40/p63 can be used (66). In addition, GATA-3 may be useful in the differential diagnosis between sarcomatoid or desmoplastic mesothelioma, both positive in most cases, and sarcomatoid carcinoma of the lung, generally negative for GATA-3 expression (67,68). Renal sarcomatoid lesions can exhibit the expression of PAX2 and PAX8 genes, but not CK5/6 protein expression (21). Synovial sarcoma is characterized by the cromosomal traslocation (X;18) and CD99 immunoreactivity (69). Indeed, other biomarkers have been tested for other sarcomas including CD31, ERG, FLI1 (angiosarcoma), CD34, STAT6 (solitary fibrous malignant tumor), desmin, myoglobin (myogenic sarcoma), S100 (liposarcoma) and SOX10 (neurogenic tumors). Muscle specific actin can be expressed in sarcomatoid mesothelioma. On the contrary, desmin expression is very rare in sarcomatoid mesothelioma but it is present in reactive mesothelial cells (21,70). Regarding the utility of BAP1 and p16 in sarcomatoid mesothelioma, the literature data are discordant and need yet to be confirmed. However, in sarcomatoid lesion, few cases have shown the loss of BAP1 expression, while homozygous deletion in the 9p21 region is seen in around 90-100% of cases (54,56,60).
Prognostic biomarkers
Some markers are studied for their prognostic value, as BAP1, P16, RTK-AXL, c-MET, p16, MDM2, WT1, p53 and EZH2 (60). Patients with BAP1 inactivation by mutation and copy number variation are younger, with epithelioid mesotheliomas and better prognosis. Multiple studies have shown that homozygous deletion of CDKN2A is associated with shorter survival in both pleural and peritoneal mesothelioma, but it is linked with increased survival post-chemotherapy. A similar prognostic value for MTAP has been assumed but has not yet been validated (60,71-73).
c-MET is an important receptor tyrosine kinase (RTK), overexpressed in some cancer types, also including PM. In a MESOPATH study, a higher c-MET expression and its localization to the membrane, compared to exclusively cytoplasmic localization or co-expression at the membrane and cytoplasm, were found to be linked with longer overall survival (74). Other biomarkers significantly associated with good prognosis, increased survival and epithelioid morphology, if overexpressed, are Axl and Syndecan-1. Axl is a RTK which is involved in cell survival and in epithelial-to-mesenchymal transition (EMT) (75). On the other hand, Syndecan acts as tumor inhibitor, preventing cellular proliferation of tumor cell, and play a role in cell adhesion and cytoskeletal organization (76). Other studies reported that neurotensin, CD9 and aquaporin are biomarkers of good prognosis, whilst increased expression of caveolin is associated with poor prognosis. In addition, diagnostic biomarkers as WT1 and calretinin, are also linked with good prognosis (60,77).
MDM2, E3 ubiquitin ligase, is an important regulator of P53 activity and stability. Overexpression of MDM2 can lead to degradation of P53 with a loss of its function and this is common in some tumors as lung, breast, colon, stomach and hepatocellular carcinomas. It is described that approximately 20% of all PM show strong nuclear MDM2 expression, especially epithelioid PM or the epithelioid component of biphasic PM. MDM2 positivity in PM is significantly associated with decreased overall survival. P14/ ARF is the physiological inhibitor of MDM2. If P14/ARF activity is lost, it may have a similar effect as loss of P53. P14/ARF is recognized as a tumor suppressor inducing cell cycle arrest in a both P53-dependent and independent manner. A recent study indicates that MDM2 mRNA and protein expression correlated significantly with overall and progression-free survival in PM, showing a poor prognosis for patients with elevated MDM2 expression. In this study, MDM2 has been indicated as a prognostic and predictive marker for a platin-pemetrexed therapy of patients with PM; at the same time, downregulation of P14/ARF expression seems to contribute to MDM2 overexpression-mediated P53 inactivation in such patients (78).
In 8% of PM it is described the loss of P53, that is important for cell protection against oxidative stress, caused by chronic inflammation related to asbestos exposure (79).
The prognostic role of EZH2 in relation to protein expression levels was examined in a study in which patients with high EZH2 expression had poorer survival (60,63). This correlation has not been confirmed by other studies (80). Therefore, more solid data on larger case histories are needed to establish the prognostic role of EZH2 (60).
New perspectives: targeted therapies and immunotherapies
The therapeutic approach for mesothelioma patients is unsatisfactory for several reasons: the diagnosis is often delayed, the tumor unresectable and the available pharmacological options are limited (4,9,81). At present, the strategies include surgery, chemotherapy and radiotherapy or a combination of them based on the clinical characteristics and stage. In patients with inoperable advanced PM, and candidates for systemic treatment, chemotherapy represents the cardinal treatment. The Food and Drug Administration (FDA) has approved cisplatin and pemetrexed for pharmacological approach with or without the addition of anti-VEGF (vascular endothelial growth factor) drugs, such as bevacizumab or nintedanib, as reported in MAPS and LUME-Meso trials respectively (82,83). As an alternative to cisplatin, carboplatin is indicated, above all, in the elderly population for the better tolerability. Generally, the epithelioid neoplastic form is less aggressive than sarcomatoid subtype, highly sensitive to chemotherapy and with a longer survival than the sarcomatoid or biphasic subtypes of PM (81,84,85).
Regarding new therapeutic options offered by molecular target therapy, phase I and phase II trials, which tested tyrosine kinase receptor inhibitors (RTK) in PM patients, with impaired regulation of EGFR and VEGFR pathways, have given poor results (86). To date, the best results are achieved with the combination of bevacizumab and chemotherapy with an increase in survival of 2.6 months compared to patients who have not received bevacizumab (87).
Deep-sequencing analysis, of 216 biopsies with PM diagnosis, has let to identify frequent gene fusions and splicing alterations causing inactivation of BAP1, NF2 and SETD2 and genetic mutations in Hippo, mTOR, p53 signaling, histone methylation and RNA helicase (88). These alterations could be new targets in the future and are being investigated. Recently, it has been demonstrated, by next-generation sequencing, high frequent homozygous inactivating mutations of the BAP1 locus in approximately 60% of mesothelioma patients (89). BAP1 gene codes for a protein binding to the breast cancer type 1 susceptibility protein (BRCA1) and the BRCA1-associated RING domain protein 1 (BARD1) (90). BAP1 is involved in many pathways including cell death, DNA damage response, homologous recombination and repair of defective DNA (91). It has been demonstrated in cell lines with loss of function of BAP1 that, by inducing synthetic lethality of alternate DNA repair pathways, poly-ADP ribose polymerase (PARP) inhibitors can cause cell death (92). The role of BAP1 in homologous recombination could be used therapeutically in a wide PM setting, similarly to what has been shown in clinical studies conducted on patients affected by ovarian or prostate cancer with mutations in the DNA repair genes. In clinical trials, the recruitment of PM patients is ongoing to examine the relationship between BAP1 genotype and response to PARP inhibitors such as olaparib (NCT03531840) and niraparib (NCT03207347) (93). The latter study is recruiting cancer patients, including those with mesothelioma, with alterations in the BAP1 gene and other deficiencies in the DNA damage response (DDR) pathway. On the contrary with what already observed in ovarian and breast cancer patients with inherited mutations in BRCA1 and BRCA2, BAP1 mutant mesothelioma cell lines resulted significantly less sensitive than BAP1 wild type cells to gemcitabine (91).
An improvement of the therapeutic possibilities comes from immunotherapy which is based on the use of checkpoint inhibitor anticancer drugs that are emerging as a front-line treatment for several types of malignancies. The most studied mechanism of checkpoint signaling is the one involving the programmed cell death-1 (PD1) receptor and its programmed cell death-1 ligand-1 (PDL1) ligand, which normally limits the activity of T lymphocytes in the tissues, subsequently to an inflammatory or autoimmune response. It is now recognized that this pathway is used in tumors to block the immune response to cancer growth since the PD1-PDL1 binding blocks the proliferation of activated T lymphocytes causing a “T-cell exhaustion” (94,95).
Recently, it has emerged that neoplastic cells, in mesothelioma, can express PDL1 protein especially in the epithelioid form and this expression is associated with a worse prognosis. In contrast, sarcomatoid mesotheliomas show PDL1 expression in a low percentage of cases that is around 30%.
Phase II clinical trials investigated PD1 and PDL1 inhibitor drugs such as pembrolizumab (already approved for NSCLC and melanoma), nivolumab, avelumab, durvalumab and CTLA4 inhibitors such as tremelimumab.
The main phase II clinical studies include the PROMISE-meso study (pembrolizumab versus second-line chemotherapy) and MAPS2 (nivolumab versus nivolumab plus ipilimumab). In monotherapy, the efficacy of pembrolizumab in unresectable patients is being analyzed in the phase Ib study, KEYNOTE-028, while the efficacy of nivolumab is being evaluated in the MERIT study. The first emerging data report a clinical benefit in a subset of patients in which there is an average increase in survival of 5–6 months (96,97). Further studies are also underway in patients with recurrence of mesothelioma.
The phase II MESOT-TREM-2008 and MESOT-TREM2012 trials have shown encouraging results and for this reason a larger randomized controlled trial, DETERMINE, has been designed with high doses of tremelimumab (98-100). The first results have been promising, as a trend has emerged in the group of sarcomatoid mesothelioma patients treated with tremelimumab. This therapeutic approach could be important for these patients characterized by a very poor prognosis. Other information comes from trials based on combined therapies such as the MAPS-II trial (nivolumab alone or nivolumab and ipilimumab) and tremelimumab with durvalumab (anti PDL1), tested on the first and second lines in the NIBT trial (101). We are waiting for the results of durvalumab together with standard chemotherapy in the phase II DREAM study (102).
Currently there are several phase III trials which have to evaluate the efficacy of immunotherapeutic approach in the first line, as Checkmate 743, IND-227 (NCT02784171) and ETOP BEAT-meso trial (NCT03762018) (93-103).
The final results of the studies mentioned above will help us to know which biomarkers will be useful to select patients with mesothelioma for immunotherapy treatment. At the present time, PDL1 is the only biomarker evaluated with an immunohistochemical assay in other neoplastic forms, and seems to allow the best selection of patients for immunotherapy. Literature data report that PDL1 is expressed in about 40-60% of mesotheliomas and when expressed it is associated with a worse prognosis. In many studies, the expression of PDL1 correlates with the response to PDL1 inhibitors with or without CTLA inhibitors (104-106). Patients treated with pembrolizumab and tumoral expression of PDL1 >1%, had response rates between 19% and 44%, while tumors with PDL1 expression <1% show clinical responses around 10% of cases (107).
However, in literature there are other studies reporting different data regarding the category of patients with PDL1 <1% which show an ORR of 27–33% or even response rates similar to those of patients with PDL1 expression >1% if treated with a combination of immunotherapy drugs (108). These data could be explained by the presence of a low number of tumor infiltrating CD8 + lymphocytes (TIL) in patients with poor response. Mesotheliomas with a high number of TILs show tumor cell apoptosis, low clinical stage and increased survival. A greater number of cases with PDL1 >1% and CD8 positive TILs are reported in the subset of sarcomatoid mesothelioma patients and this observation would explain the best response of patients with this type of mesothelioma to checkpoint inhibitor therapy (109). In conclusion, immunotherapy could represent in the near future a therapeutic option in selected groups of patients with mesothelioma, although it is necessary to identify one or more biomarkers for a wider selection of patients to be treated (110,111).
Conclusions
Mesothelioma currently remains a serious disease, not sensitive to chemotherapy, with little chance of surgical radicality which results in a low overall survival rate. The various morphological patterns require the analysis of multiple biomarkers for an accurate diagnosis. Recent and important advances in the molecular field allow the pathologist to better characterize the lesion both morphologically and genetically for address patients towards new clinical trials for innovative therapeutic possibilities.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Duilio Divisi, Roberto Crisci) for the series “Malignant Pleural Effusion” published in Journal of Xiangya Medicine. The article has undergone external peer review.
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at http://dx.doi.org/10.21037/jxym-20-79
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jxym-20-79). The series “Malignant Pleural Effusion” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ai J, Stevenson JP. Current issues in malignant pleural mesothelioma evaluation and management. Oncologist 2014;19:975-84. [Crossref] [PubMed]
- Scherpereel A, Astoul P, Baas P, et al. Guidelines of the European Respiratory Society and the European Society of Thoracic Surgeons for the management of malignant pleural mesothelioma. Eur Respir J 2010;35:479-95. [Crossref] [PubMed]
- Kim J, Bhagwandin S, Labow DM. Malignant peritoneal mesothelioma: a review. Ann Transl Med 2017;5:236. [Crossref] [PubMed]
- Novello S, Pinto C, Torri V, et al. The Third Italian consensus conference for malignant pleural mesothelioma: state of the art and recommendations. Crit Rev Oncol Hematol 2016;104:9-20. [Crossref] [PubMed]
- Pinto C, Novello S, Torri V, et al. Second Italian consensus conference on malignant pleural mesothelioma: state of the art and recommendations. Cancer Treat Rev 2013;39:328-39. [Crossref] [PubMed]
- van Zandwijk N, Clarke C, Henderson D, et al. Guidelines for the diagnosis and treatment of malignant pleural mesothelioma. J Thorac Dis 2013;5:E254-307. [PubMed]
- Carbone M, Ferris LK, Baumann F, et al. BAP1 cancer syndrome: malignant mesothelioma, uveal and cutaneous melanoma, and MBAITs. J Transl Med 2012;10:179. [Crossref] [PubMed]
- Harbour JW, Onken MD, Roberson ED, et al. Frequent mutation of BAP1 in metastasizing uveal melanomas. Science 2010;330:1410-3. [Crossref] [PubMed]
- Baas P, Fennell D, Kerr KMESMO Guidelines Committee, et al. Malignant pleural mesothelioma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2015;v31-9. [Crossref] [PubMed]
- Bray F, Ferlay M, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394-424. [Crossref] [PubMed]
- Galateau-Salle F, Churg A, Roggli V, et al. World Health Organization Committee for Tumors of the Pleura. The 2015 World Health Organization Classification of Tumors of the Pleura: Advances since the 2004 Classification. J Thorac Oncol 2016;11:142-54. [Crossref] [PubMed]
- Ascoli V, Murer B, Nottegar A, et al. What's new in mesothelioma. Pathologica 2018;110:12-28. [PubMed]
- Hjerpe A, Ascoli V, Bedrossian CW, et al. Guidelines for the cytopathologic diagnosis of epithelioid and mixed-type malignant mesothelioma: complementary statement from the International Mesothelioma Interest Group, also endorsed by the International Academy of Cytology and the Papanicolaou Society of Cytopathology. Diagn Cytopathol 2015;43:563-76. [Crossref] [PubMed]
- Rakha EA, Patil S, Abdulla K, et al. The sensitivity of cytologic evaluation of pleural fluid in the diagnosis of malignant mesothelioma. Diagn Cytopathol 2010;38:874-9. [Crossref] [PubMed]
- Sheaff M. Guidelines for the cytopathologic diagnosis of epithelioid and mixed type malignant mesothelioma: complementary statement from the international mesothelioma interest group, also endorsed by the international academy of cytology and the papanicolaou society of cytopathology. A proposal to be applauded and promoted but which requires updating. Diagn Cytopathol 2020;48:877-9. [Crossref] [PubMed]
- Churg A, Galateau-Salle F. The separation of benign and malignant mesothelial proliferations. Arch Pathol Lab Med 2012;136:1217-26. [Crossref] [PubMed]
- Cagle PT, Churg A. Differential diagnosis of benign and malignant mesothelial proliferations on pleural biopsies. Arch Pathol Lab Med 2005;129:1421-7. [PubMed]
- Husain AN, Colby TV, Ordóñez NG, et al. Guidelines for pathologic diagnosis of malignant mesothelioma 2017 update of the consensus statement from the International Mesothelioma Interest Group. Arch Pathol Lab Med 2018;142:89-108. [Crossref] [PubMed]
- Churg A, Cagle P, Colby TV, et al. The fake fat phenomenon in organizing pleuritis: a source of confusion with desmoplastic malignant mesotheliomas. Am J Surg Pathol 2011;35:1823-9. [Crossref] [PubMed]
- Pelosi G, Papotti M, Righi L, et al. Pathologic grading of malignant pleural mesothelioma. J Thorac Oncol 2018;13:1750-61. [Crossref] [PubMed]
- Alì G, Bruno R, Fontanini G. The Pathological and Molecular Diagnosis of Malignant Pleural Mesothelioma: A Literature Review. J Thorac Dis 2018;10:S276-84. [Crossref] [PubMed]
- Henderson DW, Reid G, Kao SC, et al. Challenges and controversies in the diagnosis of mesothelioma: Part 1. Cytology-only diagnosis, biopsies, immunohistochemistry, discrimination between mesothelioma and reactive mesothelial hyperplasia, and biomarkers. J Clin Pathol 2013;66:847-53. [Crossref] [PubMed]
- Hasteh F, Lin GY, Weidner N, et al. The use of immunohistochemistry to distinguish reactive mesothelial cells from malignant mesothelioma in cytologic effusions. Cancer Cytopathol 2010;118:90-6. [Crossref] [PubMed]
- King JE, Thatcher N, Pickering CA, et al. Sensitivity and specificity of immunohistochemical biomarkers used in the diagnosis of epithelioid mesothelioma: a detailed systematic analysis using published data. Histopathology 2006;48:223-32. [Crossref] [PubMed]
- Yaziji H, Battifora H, Barry TS, et al. Evaluation of 12 antibodies for distinguishing epithelioid mesothelioma from adenocarcinoma: identification of a three-antibody immunohistochemical panel with maximal sensitivity and specificity. Mod Pathol 2006;19:514-23. [Crossref] [PubMed]
- Ascoli V, Minelli G, Cozzi I, et al. Pathology reporting of malignant pleural mesothelioma first diagnosis: a population based approach. Pathol Res Pract 2016;212:886-92. [Crossref] [PubMed]
- Foster MR, Johnson JE, Olson SJ, et al. Immunohistochemical analysis of nuclear versus cytoplasmic staining of WT1 in malignant mesotheliomas and primary pulmonary adenocarcinomas. Arch Pathol Lab Med 2001;125:1316-20. [PubMed]
- Cury PM, Butcher DN, Fisher C, et al. Value of the mesothelium-associated antibodies thrombomodulin, cytokeratin 5/6, calretinin, and CD44H in distinguishing epithelioid pleural mesothelioma from adenocarcinoma metastatic to the pleura. Mod Pathol 2000;13:107-12. [Crossref] [PubMed]
- Comin CE, Novelli L, Cavazza A, et al. Expression of thrombomodulin, calretinin, cytokeratin 5/6, D2-40 and WT1 in a series of primary carcinomas of the lung: an immunohistochemical study in comparison with epithelioid pleural mesothelioma. Tumori 2014;100:559-67. [PubMed]
- Galloway ML, Murray D, Moffat DF. The use of the monoclonal antibody mesothelin in the diagnosis of malignant mesothelioma in pleural biopsies. Histopathology 2006;48:767-9. [Crossref] [PubMed]
- Mimura T, Ito A, Sakuma T, et al. Novel biomarker D2-40, combined with calretinin, CEA, and TTF-1: an optimal set of immunodiagnostic biomarkers for pleural mesothelioma. Cancer 2007;109:933-8. [Crossref] [PubMed]
- Ordóñez NG. D2-40 and podoplanin are highly specific and sensitive immunohistochemical biomarkers of epithelioid malignant mesothelioma. Hum Pathol 2005;36:372-80. [Crossref] [PubMed]
- Padgett DM, Cathro HP, Wick MR, et al. Podoplanin is a better immunohistochemical biomarker for sarcomatoid mesothelioma than calretinin. Am J Surg Pathol 2008;32:123-7. [Crossref] [PubMed]
- Chu PG, Weiss LM. Expression of cytokeratin 5/6 in epithelial neoplasms: an immunohistochemical study of 509 cases. Mod Pathol 2002;15:6-10. [Crossref] [PubMed]
- Le Stang N, Burke L, Blaizot G, et al. MESOPATH and EURACAN networks. Differential Diagnosis of Epithelioid Malignant Mesothelioma With Lung and Breast Pleural Metastasis: A Systematic Review Compared With a Standardized Panel of Antibodies-A New Proposal That May Influence Pathologic Practice. Arch Pathol Lab Med 2020;144:446-56. [Crossref] [PubMed]
- Ordóñez NG. Value of thyroid transcription factor-1, E-cadherin, BG8, WT1, and CD44S immunostaining in distinguishing epithelial pleural mesothelioma from pulmonary and non pulmonary adenocarcinoma. Am J Surg Pathol 2000;24:598-606. [Crossref] [PubMed]
- Gaffey MJ, Mills SE, Swanson PE, et al. Immunoreactivity for BEREP4 in adenocarcinomas, adenomatoid tumors, and malignant mesotheliomas. Am J Surg Pathol 1992;16:593-9. [Crossref] [PubMed]
- Oates J, Edwards C. HBME-1, MOC-31, WT1 and calretinin: an assessment of recently described biomarkers for mesothelioma and adenocarcinoma. Histopathology 2000;36:341-7. [Crossref] [PubMed]
- Bishop JA, Sharma R, Illei PB. Napsin A and thyroid transcription factor-1 expression in carcinomas of the lung, breast, pancreas, colon, kidney, thyroid, and malignant mesothelioma. Hum Pathol 2010;41:20-5. [Crossref] [PubMed]
- Ordóñez NG. The diagnostic utility of immunohistochemistry in distinguishing between epithelioid mesotheliomas and squamous carcinomas of the lung: a comparative study. Mod Pathol 2006;19:417-28. [Crossref] [PubMed]
- Tatsumori T, Tsuta K, Masai K, et al. p40 is the best marker for diagnosing pulmonary squamous cell carcinoma: comparison with p63, cytokeratin 5/6, desmocollin-3, and sox2. Appl Immunohistochem Mol Morphol 2014;22:377-82. [Crossref] [PubMed]
- Kushitani K, Amatya VJ, Okada Y, et al. Utility and pitfalls of immunohistochemistry in the differential diagnosis between epithelioid mesothelioma and poorly differentiated lung squamous cell carcinoma. Histopathology 2017;70:375-84. [Crossref] [PubMed]
- Baldovini C, Rossi G, Ciarrocchi A. Approaches to Tumor Classification in Pulmonary Sarcomatoid Carcinoma. Lung Cancer (Auckl) 2019;10:131-49. [Crossref] [PubMed]
- Ordóñez NG. The diagnostic utility of immunohistochemistry in distinguishing between mesothelioma and renal cell carcinoma: a comparative study. Hum Pathol 2004;35:697-710. [Crossref] [PubMed]
- Kaimaktchiev V, Terracciano L, Tornillo L, et al. The homeobox intestinal differentiation factor CDX2 is selectively expressed in gastrointestinal adenocarcinomas. Mod Pathol 2004;17:1392-9. [Crossref] [PubMed]
- Minato H, Kurose N, Fukushima M, et al. Comparative immunohistochemical analysis of IMP3, GLUT1, EMA, CD146, and desmin for distinguishing malignant mesothelioma from reactive mesothelial cells. Am J Clin Pathol 2014;141:85-93. [Crossref] [PubMed]
- Sheffield BS, Hwang HC, Lee AF, et al. BAP1 immunohistochemistry and p16 FISH to separate benign from malignant mesothelial proliferations. Am J Surg Pathol 2015;39:977-82. [Crossref] [PubMed]
- Carbone M, Yang H, Pass HI, et al. BAP1 and cancer. Nat Rev Cancer 2013;13:153-9. [Crossref] [PubMed]
- Andrici J, Sheen A, Sioson L, et al. Loss of expression of BAP1 is a useful adjunct, which strongly supports the diagnosis of mesothelioma in effusion cytology. Mod Pathol 2015;28:1360-8. [Crossref] [PubMed]
- Bott M, Brevet M, Taylor BS, et al. The nuclear deubiquitinase BAP1 is commonly inactivated by somatic mutations and 3p21.1 losses in malignant pleural mesothelioma. Nat Genet 2011;43:668-72. [Crossref] [PubMed]
- Yoshimura M, Kinoshita Y, Hamasaki M, et al. Diagnostic application of BAP1 immunohistochemistry to differentiate pleural mesothelioma from metastatic pleural tumours. Histopathology 2017;71:1011-4. [Crossref] [PubMed]
- Hida T, Hamasaki M, Matsumoto S, et al. BAP1 immunohistochemistry and p16 FISH results in combination provide higher confidence in malignant pleural mesothelioma diagnosis: ROC analysis of the two tests. Pathol Int 2016;66:563-70. [Crossref] [PubMed]
- Sneddon S, Leon JS, Dick IM, et al. Absence of germline mutations in BAP1 in sporadic cases of malignant mesothelioma. Gene 2015;563:103-5. [Crossref] [PubMed]
- Nasu M, Emi M, Pastorino S, et al. High incidence of somatic BAP1 alterations in sporadic malignant mesothelioma. J Thorac Oncol 2015;10:565-76. [Crossref] [PubMed]
- Righi L, Duregon E, Vatrano S, et al. BRCA1-associated protein 1 (BAP1) immunohistochemical expression as a diagnostic tool in malignant pleural mesothelioma classification: a large retrospective study. J Thorac Oncol 2016;11:2006-17. [Crossref] [PubMed]
- Ladanyi M. Implications of P16/CDKN2A deletion in pleural mesotheliomas. Lung Cancer 2005;49:S95-8. [Crossref] [PubMed]
- Monaco S, Mehrad M, Dacic S. Recent Advances in the Diagnosis of Malignant Mesothelioma: Focus on Approach in Challenging Cases and in Limited Tissue and Cytologic Samples. Adv Anat Pathol 2018;25:24-30. [Crossref] [PubMed]
- Bruno R, Alì G, Fontanini G. Molecular markers and new diagnostic methods to differentiate malignant from benign mesothelial pleural proliferations: a literature review. J Thorac Dis 2018;10:S342-52. [Crossref] [PubMed]
- Hida T, Hamasaki M, Matsumoto S, et al. Immunohistochemical detection of MTAP and BAP1 protein loss for mesothelioma diagnosis: Comparison with 9p21 FISH and BAP1 immunohistochemistry. Lung Cancer 2017;104:98-105. [Crossref] [PubMed]
- Chapel DB, Schulte JJ, Husain AN, et al. Application of immunohistochemistry in diagnosis and management of malignant mesothelioma. Transl Lung Cancer Res 2020;9:S3-27. [Crossref] [PubMed]
- Chapel DB, Husain AN, Krausz T. Immunohistochemical evaluation of nuclear 5-hydroxymethylcytosine (5-hmC) accurately distinguishes malignant pleural mesothelioma from benign mesothelial proliferations. Mod Pathol 2019;32:376-86. [Crossref] [PubMed]
- Yoshimura M, Kinoshita Y, Hamasaki M, et al. Highly expressed EZH2 in combination with BAP1 and MTAP loss, as detected by immunohistochemistry, is useful for differentiating malignant pleural mesothelioma from reactive mesothelial hyperplasia. Lung Cancer 2019;130:187-93. [Crossref] [PubMed]
- LaFave LM, Béguelin W, Koche R, et al. Loss of BAP1 function leads to EZH2-dependent transformation. Nat Med 2015;21:1344-9. [Crossref] [PubMed]
- Lucas DR, Pass HI, Madan SK, et al. Sarcomatoid mesothelioma and its histological mimics: a comparative immunohistochemical study. Histopathology 2003;42:270-9. [Crossref] [PubMed]
- Mangano WE, Cagle PT, Churg A, et al. The diagnosis of desmoplastic malignant mesothelioma and its distinction from fibrous pleurisy: a histologic and immunohistochemical analysis of 31 cases including p53 immunostaining. Am J Clin Pathol 1998;110:191-9. [Crossref] [PubMed]
- Beasley MB. Immunohistochemistry of pulmonary and pleural neoplasia. Arch Pathol Lab Med 2008;132:1062-72. [PubMed]
- Shinozaki-Ushiku A, Ushiku T, Morita S, et al. Diagnostic utility of BAP1 and EZH2 expression in malignant mesothelioma. Histopathology 2017;70:722-33. [Crossref] [PubMed]
- Ettinger DS, Wood DE, Akerley W, et al. NCCN Guidelines Insights: Malignant Pleural Mesothelioma, Version 3.2016. J Natl Compr Canc Netw 2016;14:825-36. [Crossref] [PubMed]
- Miettinen M, Limon J, Niezabitowski A, et al. Calretinin and other mesothelioma markers in synovial sarcoma: analysis of antigenic similarities and differences with malignant mesothelioma. Am J Surg Pathol 2001;25:610-7. [Crossref] [PubMed]
- Rdzanek M, Fresco R, Pass HI, et al. Spindle cell tumors of the pleura: differential diagnosis. Semin Diagn Pathol 2006;23:44-55. [Crossref] [PubMed]
- Farzin M, Toon CW, Clarkson A, et al. Loss of expression of BAP1 predicts longer survival in mesothelioma. Pathology 2015;47:302-7. [Crossref] [PubMed]
- Baumann F, Flores E, Napolitano A, et al. Mesothelioma patients with germline BAP1 mutations have 7-fold improved long-term survival. Carcinogenesis 2015;36:76-81. [Crossref] [PubMed]
- Davidson B. Prognostic factors in malignant pleural mesothelioma. Hum Pathol 2015;46:789-804. [Crossref] [PubMed]
- Levallet G, Vaisse-Lesteven M, Le Stang N, et al. Plasma cell membrane localization of c-MET predicts longer survival in patients with malignant mesothelioma: a series of 157 cases from the MESOPATH Group. J Thorac Oncol 2012;7:599-606. [Crossref] [PubMed]
- Pinato DJ, Mauri FA, Lloyd T, et al. The expression of Axl receptor tyrosine kinase influences the tumour phenotype and clinical outcome of patients with malignant pleural mesothelioma. Br J Cancer 2013;108:621-8. [Crossref] [PubMed]
- Kumar-Singh S, Jacobs W, Dhaene K, et al. Syndecan-1 expression in malignant mesothelioma: correlation with cell differentiation, WT1 expression, and clinical outcome. J Pathol 1998;186:300-5. [Crossref] [PubMed]
- Cedrés S, Montero MA, Zamora E, et al. Expression of Wilms’ tumor gene (WT1) is associated with survival in malignant pleural mesothelioma. Clin Transl Oncol 2014;16:776-82. [Crossref] [PubMed]
- Walter RF, Mairinger FD, Ting S, et al. MDM2 is an important prognostic and predictive factor for platin-pemetrexed therapy in malignant pleural mesotheliomas and deregulation of P14/ARF (encoded by CDKN2A) seems to contribute to an MDM2-driven inactivation of P53. Br J Cancer 2015;112:883-90. [Crossref] [PubMed]
- Tian K, Bakker E, Hussain M, et al. p53 modeling as a route to mesothelioma patients stratification and novel therapeutic identification. J Transl Med 2018;16:282. [Crossref] [PubMed]
- Blyth K G, Murphy D J. Progress and Challenges in Mesothelioma: From Bench to Bedside. Respir Med 2018;134:31-41. [Crossref] [PubMed]
- Rossini M, Rizzo P, Bononi I, et al. New Perspectives on Diagnosis and Therapy of Malignant Pleural Mesothelioma. Front Oncol 2018;8:91. [Crossref] [PubMed]
- Scherpereel A, Mazieres J, Greillier L, et al. Nivolumab or nivolumab plus ipilimumab in patients with relapsed malignant pleural mesothelioma (IFCT-1501 MAPS2): a multicentre, openlabel, randomised, non-comparative, phase 2 trial. Lancet Oncol 2019;20:239-53. [Crossref] [PubMed]
- Scagliotti GV, Gaafar R, Nowak AK, et al. Nintedanib in combination with pemetrexed and cisplatin for chemotherapy-naive patients with advanced malignant pleural mesothelioma (LUME-Meso): a double-blind, randomised, placebo-controlled phase 3 trial. Lancet Respir Med 2019;7:569-80. [Crossref] [PubMed]
- Brims FJ, Meniawy TM, Duffus I, et al. A novel clinical prediction model for prognosis in malignant pleural mesothelioma using decision tree analysis. J Thorac Oncol 2016;11:573-82. [Crossref] [PubMed]
- Billé A, Krug LM, Woo KM, et al. Contemporary analysis of prognostic factors in patients with unresectable malignant pleural mesothelioma. J Thorac Oncol 2016;11:249-55. [Crossref] [PubMed]
- Guazzelli A, Bakker E, Tian K, et al. Promising investigational drug candidates in phase I and phase II clinical trials for mesothelioma. Expert Opin Investig Drugs 2017;26:933-44. [Crossref] [PubMed]
- Zalcman G, Mazieres J, Margery J, et al. Bevacizumab for newly diagnosed pleural mesothelioma in the Mesothelioma Avastin Cisplatin Pemetrexed Study (MAPS): a randomised, controlled, open-label, phase 3 trial. Lancet 2016;387:1405-14. [Crossref] [PubMed]
- Bueno R, Stawiski EW, Goldstein LD, et al. Comprehensive genomic analysis of malignant pleural mesothelioma identifies recurrent mutations, gene fusions and splicing alterations. Nat Genet 2016;48:407-16. [Crossref] [PubMed]
- Yoshikawa Y, Emi M, Hashimoto-Tamaoki T, et al. High-density array-CGH with targeted NGS unmask multiple noncontiguous minute deletions on chromosome 3p21 in mesothelioma. Proc Natl Acad Sci USA 2016;113:13432-7. [Crossref] [PubMed]
- Jensen DE, Proctor M, Marquis ST, et al. BAP1: A novel ubiquitin hydrolase which binds to the BRCA1 RING finger and enhances BRCA1-mediated cell growth suppression. Oncogene 1998;16:1097-112. [Crossref] [PubMed]
- Guazzelli A, Meysami P, Bakker E, et al. BAP1 status determines the sensitivity of malignant mesothelioma cells to gemcitabine treatment. Int J Mol Sci 2019;20:429. [Crossref] [PubMed]
- Pinton G, Manente AG, Murer B, et al. PARP1 inhibition affects pleural mesothelioma cell viability and uncouples AKT/mTOR axis via SIRT1. J Cell Mol Med 2013;17:233-241. [Crossref] [PubMed]
- ClinicalTrials provided by the U.S. National Library of Medicine. Avalilable online: www.cllinicaltrials.gov
- de Gooijer CJ, Borm FJ, Scherpereel A, et al. Immunotherapy in Malignant Pleural Mesothelioma. Front Oncol 2020;21;10:187.
- Minchom A, Yuan W, Crespo M, et al. Molecular and immunological features of a prolonged exceptional responder with malignant pleural mesothelioma treated initially and rechallenged with pembrolizumab. J Immunother Cancer 2020;8:e000713. [Crossref] [PubMed]
- Alley EW, Lopez J, Santoro A, et al. Clinical safety and activity of pembrolizumab in patients with malignant pleural mesothelioma (KEYNOTE-028): preliminary results from a non randomised, open-label, phase 1b trial. Lancet Oncol 2017;18:623-30. [Crossref] [PubMed]
- Okada M, Kijima T, Aoe K, et al. Clinical Efficacy and Safety of Nivolumab: Results of a Multicenter, Open-label, Single-arm, Japanese Phase II study in Malignant Pleural Mesothelioma (MERIT). Clin Cancer Res 2019;25:5485-92. [Crossref] [PubMed]
- Calabrò L, Morra A, Fonsatti E, et al. Tremelimumab for patients with chemotherapy-resistant advanced malignant mesothelioma: an open-label, single-arm, phase 2 trial. Lancet Oncol 2013;14:1104-11. [Crossref] [PubMed]
- Calabrò L, Morra A, Fonsatti E, et al. Efficacy and safety of an intensified schedule of tremelimumab for chemotherapy resistant malignant mesothelioma: an open-label, single-arm, phase 2 study. Lancet Respir Med 2015;3:301-9. [Crossref] [PubMed]
- Maio M, Scherpereel A, Calabrò L, et al. Tremelimumab as second-line or third-line treatment in relapsed malignant mesothelioma (DETERMINE): a multicentre, international, randomised, double-blind, placebo-controlled phase2b trial. Lancet Oncol 2017;18:1261-73. [Crossref] [PubMed]
- Calabrò L, Morra A, Giannarelli D, et al. Tremelimumab combined with durvalumab in patients with mesothelioma (NIBIT-MESO-1): an open-label, non-randomised, phase 2 study. Lancet Respir Med 2018;6:451-60. [Crossref] [PubMed]
- Nowak A, Kok P, Lesterhuis W, et al. OA08.02 dream - a phase 2 trial of durvalumab with first line chemotherapy in mesothelioma: final result. J Thoracic Oncol 2018;13:S338-9. [Crossref]
- Popat S, Curioni-Fontecedro A, Polydoropoulou V, et al. LBA91_PRA multicentre randomized phase III trial comparing pembrolizumab (P) vs single agent chemotherapy (CT) for advanced pre-treated malignant pleural mesothelioma (MPM): results from the European Thoracic Oncology Platform (ETOP 9-15) PROMISE-meso trial. Annal Oncol 2019;30:mdz394.001.
- Metaxas Y, Rivalland G, Mauti LA, et al. Pembrolizumab as palliative immunotherapy in malignant pleural mesothelioma. J Thorac Oncol 2018;13:1784-91. [Crossref] [PubMed]
- Marcq E, Siozopoulou V, De Waele J, et al. Prognostic and predictive aspects of the tumor immune microenvironment and immune checkpoints in malignant pleural mesothelioma. Oncoimmunology 2016;6:e1261241. [Crossref] [PubMed]
- Scherpereel A, Wallyn F, Albelda SM, et al. Novel therapies for malignant pleural mesothelioma. Lancet Oncol 2018;19:e161-72. [Crossref] [PubMed]
- Metaxas Y, Rivalland G, Mauti LA, et al. Pembrolizumab as Palliative Immunotherapy in Malignant Pleural Mesothelioma. J Thorac Oncol 2018;13:1784-91. [Crossref] [PubMed]
- Chatwal MS, Tanvetyanon T. Malignant mesothelioma clinical trial combines immunotherapy drugs. Immunotherapy 2018;10:341-4. [Crossref] [PubMed]
- Losi L, Bertolini F, Guaitoli G, et al. Role of evaluating tumor infiltrating lymphocytes, programmed death 1 ligand 1 and mismatch repair proteins expression in malignant mesothelioma. Int J Oncol 2019;55:1157-64. [Crossref] [PubMed]
- Cantini L, Hassan R, Sterman DH, et al. Emerging Treatments for Malignant Pleural Mesothelioma: Where Are We Heading? Front Oncol 2020;10:343. [Crossref] [PubMed]
- Opitz I, Scherpereel A, Berghmans T, et al. ERS/ESTS/EACTS/ESTRO guidelines for the management of malignant pleural mesothelioma. Eur J Cardiothorac Surg 2020;58:1-24. [Crossref] [PubMed]
Cite this article as: Buttitta F, Di Lorito A, D’Angelo E. Biomarkers in the diagnosis and treatment of malignant pleural disease: an integrative review. J Xiangya Med 2020;5:40.


