电视胸腔镜在恶性胸膜疾病的诊断和治疗中的应用
简介
恶性胸腔积液常发生于转移性肿瘤,也发生于胸膜原发疾病。除了直接扩散外,肿瘤细胞可通过血液和淋巴通道转移,这可能是恶性胸腔积液产生的机制。肺癌是引起胸腔积液的主要原因,约占35%;第二个与恶性胸腔积液产生相关联的癌种是乳腺癌。但是,超过7%的恶性胸腔积液的病因是不明的[1]。恶性胸腔积液的实际发生率不太确定,但美国每年大约有15万例新诊断的患者[2]。电视胸腔镜手术(VATS)和胸膜固定术被广泛应用,但是,它们没有统一的实施方法[3]。
本文章综述了VATS应用于恶性胸腔积液的诊断过程和治疗方式。
方法
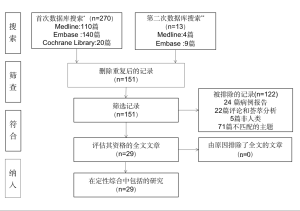
我们从Medline、Embase和Cochrane Library搜索了相关文章,主题词为:(胸腔积液,恶性)和(((VATS)或胸外科,电视辅助)或“局部麻醉胸腔镜”)。搜索仅限制在近10年以英文发表的文献;临床病例报告被排除在外。下载搜索结果;获取摘要和全文,以确定潜在的相关研究。纳入标准为随机试验、前瞻性和回顾性队列研究。排除标准为综述论文和评论。
在同一数据库中进行了第二次搜索,主题词为胸腔积液。该搜索仅限于过去10年发表的英文指南。同时,还进行了人工搜索。
结果
第一次搜索共得到151个结果,其中25篇文章符合纳入标准。其中随机性研究[4-8]、前瞻性研究[9-17]、回顾性研究[18-28]的数量分别为5篇、9篇、11篇。第二次搜索确定了3篇指南[29-31]和1项欧洲研究[32]。图1为PRISMA流程。
讨论
虽然恶性胸腔积液在胸外科医生的日常工作中时常发生,但是文献中并没有丰富的科学性文章和指南。英国指南推荐,对成年人的单侧胸腔积液,推荐胸腔穿刺作为首要诊断方式[30]。胸腔穿刺术诊断率取决于影响胸膜的恶性肿瘤类型;细胞学检查的敏感性在腺癌中相对较高(57%~83%),但是在胸膜间皮瘤中敏感性非常有限(20%)[33]。因此,对胸腔积液患者行细胞学检查,许多患者依然不能确诊恶性胸腔积液。
肿瘤分子检查,特别是在肺腺癌中,已经成为预测靶向治疗疗效的必需过程。最近的试验评估了新一代测序技术的诊断性能,以实现对胸腔积液的致癌基因分析,结果令人鼓舞[34]。但考虑到更多的肿瘤组织可用于分子检测,手术活检保证了更好的诊断充分性[35]。
最新的基因研究显示,转移肿瘤与原始肿瘤表现出不同的特定特征[36]。转移肿瘤中的基因组、表观遗传学和其他分子变化可能会影响治疗策略;因此,肿瘤学家越来越需要对已知的原发肿瘤的转移瘤进行活检。
考虑到超过40%胸腔积液的细胞检查是不足的,因此,分子检测的组织标准需求不断增加。对于恶性胸腔积液患者,可通过内镜手术这种简单的方式取得胸膜组织进行诊断,同时进行肿瘤治疗。
胸膜镜手术
胸膜镜手术或胸腔镜手术首次报道是在20世纪早期。在患者适度镇静、自主呼吸的情况下,介入性胸外科医生使用配备活检钳的刚性或半刚性胸膜镜进行操作。最近,加拿大学者[19]发布了一项回顾性研究,比较胸膜镜手术和VATS在胸膜活检诊断上的价值。VATS操作者进行单腔及单肺通气手术。胸膜镜检查和VATS手术的活检诊断准确率大约分别为93.5%和96%。该作者认为胸膜腔镜检查和VATS手术具有相同的诊断率和安全特性。但是,胸膜镜手术比VATS便宜,因此,该作者认为胸膜镜手术可作为在选定的条件下的首选技术。
VATS
VATS被认为是胸膜镜手术的另一个版本。在过去的20年中,胸外科医生可通过VATS完成复杂的手术操作。胸外科医生认为胸膜活检是VATS可进行的最简单的手术,因此,胸膜活检常作为训练者的首个操作过程。
近年来,出现了一种胸膜镜和胸腔镜之间的综合性操作:非插管和清醒下的VATS。该技术最小化了双肺通气和单肺通气的缺点。尽管这个技术主要用于肺部手术,但是对于胸腔积液的手术处理同样具有很大的吸引力。最近的27项研究显示,相对于传统的气管插管VATS,非气管插管VATS肺部炎症反应和免疫刺激减少,加快了康复,同时减少了花费[37]。
从技术的观点来看,进入胸腔的数量和质量会影响术后恢复。一项小型但有趣的随机性研究分析显示切口数量对术后恢复并无影响[5]。最近保加利亚进行了一项前瞻性分析,研究对胸腔积液患者而言,VATS的切口数目是否对疼痛和患者满意度有影响[9]。该作者得出结论,单孔比三孔VATS效果更好。值得注意的是,这个是胸外科医生讨论得最多的问题之一,但最终决议仍没有达成一致。
有时候,疑似恶性胸腔积液患者的胸膜显示是正常的,从这一方面而言,帮助识别可能的转移位置是很重要的;为了实现这种需求,一些学者测试了荧光技术。俄罗斯研究人员用5-氨基乙酰丙酸对胸膜恶性肿瘤进行了荧光检测,而自体荧光似乎能比胸膜原发肿瘤更好地识别胸膜转移[17,25]。
滑石粉胸膜固定术
白求恩(Bethune)在1930年首次报道了“碘化”滑石粉的使用;他的目的是形成胸膜粘连,“作为肺叶切除术的初步准备”。
以胸膜固定术为目的,滑石粉可通过胸腔镜(滑石粉)或者胸管(滑石浆)使用。尽管已确定滑石粉是一个很好的硬化剂,但依然需要讨论滑石粉和滑石浆哪一个更有效[31]。一项由英国学者在2019年年底发表的随机性试验,研究了两种滑石粉给药的方法[38]。共有17家医院、330例患者参加了TAPPS试验(比较胸腔镜喷洒滑石粉和胸管注入滑石浆胸膜固定术的疗效)。在滑石粉固定术和滑石浆固定术中,胸膜固定的成功率分别约为22%和24%。尽管研究者认为该试验效力不足,但他们认为在3个月内,滑石浆和滑石粉在胸膜固定成功率上并无明显差异。
滑石粉胸膜固定术的替代方案
英国研究人员在2014年发表了Meso VATS试验结果[6]。该随机试验目的是确定与胸膜固定术相比,VATS部分胸膜切除术是否能改善恶性胸膜间皮瘤继发胸腔积液患者的生存率、胸腔积液和生活质量。不幸的是,在研究的10年中,分期系统、手术技术和新引进的化疗技术均发生了改变。最终,VATS组和滑石粉胸膜固定术组的中位生存期约为13.1个月和13.5个月。另一方面,VATS组的生活质量明显改善。
最近,希腊学者发表了一项随机性对照研究,旨在比较恶性胸腔积液和肺癌患者行胸腔热灌注和滑石粉喷洒后的生存率[4]。实验组(20例)使用41.5℃的卡铂(500 mg/m2)进行胸膜灌注45分钟。胸膜灌注和滑石粉胸膜固定组的中位生存期分别约为8个月和9个月。
Mohsen等[8]发表了一项随机试验,比较了床边使用10%聚维酮碘溶液和VATS滑石粉浆的有效性和安全性。结论为两者治疗效果相同。值得注意的是,研究对于胸膜腔内给碘溶液的安全性存在一些担忧。
最近发表的Cochrane Library网络Meta分析,其重点是确定最佳胸膜固定术治疗剂[39]。作者评估了55篇文章,报告了21个不同的药物或给药程序。滑石粉比博来霉素、四环素、莫司汀、干扰素、留置胸腔导管、米托蒽醌和安慰剂有更高的胸膜固定成功率。最终,作者将滑石粉与滑石浆在低偏倚风险研究下进行了比较;两种方法的成功率相同。
局限性
目前的综述具有局限性;正如每一项叙述性回顾,本综述包含偏倚风险。我们试图通过报告最近的系统性研究的结果,来减轻这种影响。我们的搜索策略因限制在过去10年的时间框架,可能还没有确定所有相关的临床研究。另一方面,我们的目的是回顾最新的研究,以突出任何问题。尽管恶性胸腔积液是常见的临床问题,但许多研究的样本量相对较小,这可能会影响相关的结果。最后,目前的综述必然在证据的寻找、纳入研究的相关性及其有效性方面模糊不清。
结论
恶性胸腔积液常影响转移患者的临床进展。在靶向治疗的时代,必须正确诊断低风险患者的胸膜转移,即使原发肿瘤是明确的。胸膜活检依然是恶性胸腔积液诊断的金标准。胸膜活检可通过胸膜镜或VATS进行;对这两种方式的选择应根据当地医疗水平决定。非插管的VATS是一个很好的折中方案,结合了手术入路的技巧和避免全身麻醉的优点。VATS中套管针的类型和数量似乎对短期预后没有影响。最好的预防胸腔积液积累的方法是滑石粉胸膜固定术。如果在胸膜镜手术或VATS期间使用滑石粉,任何积液复发都不支持采用新的手术方法,因为滑石浆同样有效。
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Duilio Divisi, Roberto Crisci) for the series “Malignant Pleural Effusion” published in Journal of Xiangya Medicine. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jxym-20-52). The series “Malignant Pleural Effusion” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Awadallah SF, Bowling MR, Sharma N, et al. Malignant pleural effusion and cancer of unknown primary site: a review of literature. Ann Transl Med 2019;7:353. [Crossref] [PubMed]
- American Thoracic Society. Management of malignant pleural effusions. Am J Respir Crit Care Med 2000;162:1987-2001. [Crossref] [PubMed]
- Perikleous P, Waller DA. Video assisted thoracoscopic and open chest surgery in diagnosis and treatment of malignant pleural diseases. J Vis Surg 2017;3:85. [Crossref] [PubMed]
- Kleontas A, Sioga A, Pandria N, et al. Clinical factors affecting the survival of patients diagnosed with non-small cell lung cancer and metastatic malignant pleural effusion, treated with hyperthermic intrathoracic chemotherapy or chemical talc pleurodesis: a monocentric, prospective, randomized trial. J Thorac Dis 2019;11:1788-98. [Crossref] [PubMed]
- Dell'Amore A, Campisi A, Giunta D, et al. The influence of the trocar choice on post-operative acute pain after thoracoscopy. J Vis Surg 2018;4:104. [Crossref] [PubMed]
- Rintoul RC, Ritchie AJ, Edwards JG, et al. Efficacy and cost of video-assisted thoracoscopic partial pleurectomy versus talc pleurodesis in patients with malignant pleural mesothelioma (MesoVATS): an open-label, randomised, controlled trial. Lancet 2014;384:1118-27. [Crossref] [PubMed]
- Son HS, Lee SH, Darlong LM, et al. Is There a Role for a Needle Thoracoscopic Pleural Biopsy under Local Anesthesia for Pleural Effusions? Korean J Thorac Cardiovasc Surg 2014;47:124-8. [Crossref] [PubMed]
- Mohsen TA, Zeid AA, Meshref M, et al. Local iodine pleurodesis versus thoracoscopic talc insufflation in recurrent malignant pleural effusion: a prospective randomized control trial. Eur J Cardiothorac Surg 2011;40:282-6. [PubMed]
- Valchev D, Peeva K, Petrov D. Are postoperative pain and patient satisfaction influenced by the number of ports in VATS for malignant pleural effusion treatment? Postgrad Med 2020;132:62-5. [Crossref] [PubMed]
- Feng X, Zhu L, Xiong X, et al. Therapeutical effect of intrapleural perfusion with hyperthermic chemotherapy on malignant pleural effusion under video-assisted thoracoscopic surgery. Int J Hyperthermia 2018;34:479-85. [Crossref] [PubMed]
- Sakaguchi H, Ishida H, Nitanda H, et al. Pharmacokinetic evaluation of intrapleural perfusion with hyperthermic chemotherapy using cisplatin in patients with malignant pleural effusion. Lung Cancer 2017;104:70-4. [Crossref] [PubMed]
- Walker S, Zubrinic M, Massey C, et al. A prospective study of patient-centred outcomes in the management of malignant pleural effusions. Int J Palliat Nurs 2016;22:351-8. [Crossref] [PubMed]
- Kara M, Alzafer S, Okur E, et al. The use of single incision thoracoscopic pleurectomy in the management of malignant pleural effusion. Acta Chir Belg 2013;113:270-4. [Crossref] [PubMed]
- Alar T, Ozcelik C. Single-incision thoracoscopic surgery of pleural effusions for diagnosis and treatment. Surg Endosc 2013;27:4333-6. [Crossref] [PubMed]
- Basso SM, Mazza F, Marzano B, et al. Improved quality of life in patients with malignant pleural effusion following video-assisted thoracoscopic talc pleurodesis. Preliminary results. Anticancer Res 2012;32:5131-4. [PubMed]
- Hunt BM, Farivar AS, Vallières E, et al. Thoracoscopic talc versus tunneled pleural catheters for palliation of malignant pleural effusions. Ann Thorac Surg 2012;94:1053-7. [Crossref] [PubMed]
- Pikin O, Filonenko E, Mironenko D, et al. Fluorescence thoracoscopy in the detection of pleural malignancy. Eur J Cardiothorac Surg 2012;41:649-52. [Crossref] [PubMed]
- Dadaş E, Erdoğdu E, Toker A, et al. Effectiveness of Video-Assisted Thoracoscopic Surgery in Undiagnosed Exudative Pleural Effusions. Turk Thorac J 2019;20:188-91. [Crossref] [PubMed]
- McDonald CM, Pierre C, de Perrot M, et al. Efficacy and Cost of Awake Thoracoscopy and Video-Assisted Thoracoscopic Surgery in the Undiagnosed Pleural Effusion. Ann Thorac Surg 2018;106:361-7. [Crossref] [PubMed]
- Hu R, Jiang H, Li H, et al. Intrapleural perfusion thermo-chemotherapy for pleural effusion caused by lung carcinoma under VATS. J Thorac Dis 2017;9:1317-21. [Crossref] [PubMed]
- Yoon DW, Cho JH, Choi YS, et al. Predictors of survival in patients who underwent video-assisted thoracic surgery talc pleurodesis for malignant pleural effusion. Thorac Cancer 2016;7:393-8. [Crossref] [PubMed]
- Marchetti G, Valsecchi A, Indellicati D, et al. Ultrasound-guided medical thoracoscopy in the absence of pleural effusion. Chest 2015;147:1008-12. [Crossref] [PubMed]
- Mineo TC, Sellitri F, Tacconi F, et al. Quality of life and outcomes after nonintubated versus intubated video-thoracoscopic pleurodesis for malignant pleural effusion: comparison by a case-matched study. J Palliat Med 2014;17:761-8. [Crossref] [PubMed]
- Freeman RK, Ascioti AJ, Mahidhara RS. A propensity-matched comparison of pleurodesis or tunneled pleural catheter in patients undergoing diagnostic thoracoscopy for malignancy. Ann Thorac Surg 2013;96:259-63: discussion 263-4.
- Liman ST, Elicora A, Topcu S, et al. Value of autofluorescence in video-assisted thoracoscopic surgery in pleural diseases. Thorac Cardiovasc Surg 2013;61:350-6. [Crossref] [PubMed]
- Whitworth JM, Schneider KE, Fauci JM, et al. Outcomes of patients with gynecologic malignancies undergoing video-assisted thoracoscopic surgery (VATS) and pleurodesis for malignant pleural effusion. Gynecol Oncol 2012;125:646-8. [Crossref] [PubMed]
- Sayir F, Cobanoglu U, Mergan D, et al. Video-assisted thoracoscopic surgery for malignant pleural effusions. Asian Pac J Cancer Prev 2011;12:415-8. [PubMed]
- Barbetakis N, Asteriou C, Papadopoulou F, et al. Early and late morbidity and mortality and life expectancy following thoracoscopic talc insufflation for control of malignant pleural effusions: a review of 400 cases. J Cardiothorac Surg 2010;5:27. [Crossref] [PubMed]
- Bibby AC, Dorn P, Psallidas I, et al. ERS/EACTS statement on the management of malignant pleural effusions. Eur J Cardiothorac Surg 2019;55:116-32. [Crossref] [PubMed]
- Hooper C, Lee YC, Maskell N. Investigation of a unilateral pleural effusion in adults: British Thoracic Society Pleural Disease Guideline 2010. Thorax 2010;65:ii4-ii17. [Crossref] [PubMed]
- Roberts ME, Neville E, Berrisford RG, et al. Management of a malignant pleural effusion: British Thoracic Society Pleural Disease Guideline 2010. Thorax 2010;65:ii32-ii40. [Crossref] [PubMed]
- Scarci M, Caruana E, Bertolaccini L, et al. Current practices in the management of malignant pleural effusions: a survey among members of the European Society of Thoracic Surgeons. Interact Cardiovasc Thorac Surg 2017;24:414-7. [PubMed]
- Rodríguez-Panadero F. Medical thoracoscopy. Respiration 2008;76:363-72. [Crossref] [PubMed]
- Liu L, Shao D, Deng Q, et al. Next generation sequencing-based molecular profiling of lung adenocarcinoma using pleural effusion specimens. J Thorac Dis 2018;10:2631-7. [Crossref] [PubMed]
- Cooke DT, Gandara DR, Goodwin NC, et al. Outcomes and efficacy of thoracic surgery biopsy for tumor molecular profiling in patients with advanced lung cancer. J Thorac Cardiovasc Surg 2014;148:36-40. [Crossref] [PubMed]
- Allgayer H, Leupold JH, Patil N. Defining the "Metastasome": Perspectives from the genome and molecular landscape in colorectal cancer for metastasis evolution and clinical consequences. Semin Cancer Biol 2020;60:1-13. [Crossref] [PubMed]
- Yu MG, Jing R, Mo YJ, et al. Non-intubated anesthesia in patients undergoing video-assisted thoracoscopic surgery: A systematic review and meta-analysis. PLoS One 2019;14:e0224737. [Crossref] [PubMed]
- Bhatnagar R, Piotrowska HEG, Laskawiec-Szkonter M, et al. Effect of Thoracoscopic Talc Poudrage vs Talc Slurry via Chest Tube on Pleurodesis Failure Rate Among Patients With Malignant Pleural Effusions: A Randomized Clinical Trial. JAMA 2019;323:60-9. [Crossref] [PubMed]
- Dipper A, Jones HE, Bhatnagar R, et al. Interventions for the management of malignant pleural effusions: a network meta-analysis. Cochrane Database Syst Rev 2020;4:CD010529. [PubMed]

阮英定
建德市第一人民医院,研究生学历,胸外科专业,规培医院为大连大学附属中山医院。2019年顺利毕业。毕业后在建德市第一人民医院工作,从事胸外科。近5年在《中华胸部外科电子杂志》上发表论文2篇。对肺癌的相关研究,特别对小细胞肺癌的后续研究特别感兴趣。(更新时间:2021/8/12)
(本译文仅供学术交流,实际内容请以英文原文为准。)
Cite this article as: Nosotti M, Mazzucco A, Daffrè E, Cattaneo M, Ferrari M, Mendogni P. Video-assisted thoracoscopy in the diagnosis and treatment of malignant pleural disease. J Xiangya Med 2020;5:27.