
Multidisciplinary team approach on a case of bilateral interstitial pneumonia
IntroductionOther Section
- Introduction
- Case presentation
- iMDT discussion
- Several issues regarding the diagnosis and treatment of this patient were further discussed as follows
- Conclusions
- Acknowledgments
- Footnote
- References
Hypersensitivity pneumonitis (HP) is an interstitial lung disease (ILD) caused by exposure to a wide variety of organic particles that cause an excessive immune response to small airways and lung parenchyma (1). Distinguishing HP from other ILDs is a diagnostic challenge because of the absence of any unique features. HP may present as acute, subacute, or chronic clinical forms but with frequent overlap of these various forms (2). Sulfasalazine is a commonly used drug for the treatment of inflammatory bowel disease (IBD). Lung parenchymal diseases are reported to be induced by drugs such as sulfasalazine in IBD, on the other hand, IBD itself may also induce ILD that make this clinical problem become very complicated. In this article, we report a rare case of chronic interstitial pneumonia, whose imaging and pathology are consistent with chronic hypersensitivity pneumonia, and may be related to the use of sulfasalazine or IBD itself, and further diagnosis and treatment are discussed.
Case presentationOther Section
- Introduction
- Case presentation
- iMDT discussion
- Several issues regarding the diagnosis and treatment of this patient were further discussed as follows
- Conclusions
- Acknowledgments
- Footnote
- References
A 44-year-old male patient with cough and shortness of breath for more than 4 years and exacerbation for one month without confirmed diagnosis came to Xiangya Hospital of Central South University for further treatment in August 2018. In 2016, according to the results of chest high-resolution computerized tomography (HRCT) and transbronchial lung biopsy (TBLB), the patient was considered to have idiopathic interstitial pneumonia (undifferentiated type) and received symptomatic treatment. Yet no obvious relief was observed. During the last one and a half years, he visited a number of hospitals. However, he gradually suffered from aggravated cough and shortness of breath on exertion despite intermittent medications. He even had difficulty in walking within the last month.
The patient had a history of ulcerative colitis for 5 years, and used to take salazosulfapyridine 0.75 g 3 times a day for more than 4 years from 2013. It was changed to 0.5 g 3 times a day half a year ago with colonoscopy confirming his improvement. The patient had no history of hypertension, coronary artery disease, tuberculosis (TB) or pollen hypersensitivity. He had no exposure to any toxic substance or special occupational environment.
As for physical examination, the body temperature was 36.7 °C, respiratory rate (RR) was 21 breaths per minute, pulse rate was 85 beats per minute, blood pressure was 110/60 mmHg, peripheral oxygen saturation (SpO2) was 90% (oxygen concentration was 33%), body height was 175 cm, and body weight was 65 kg. The lips were cyanotic. Barrel chest and hyperpnoea were noticed, with mild dry and moist rales. The heart rhythm was regular and no obvious pathological murmur was noticed. Acropachy was observed.
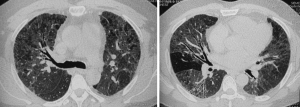
Chest computed tomography (CT) revealed bilateral diffuse interstitial lung lesions including ground-glass opacities, small nodules, and thickening of the interlobular septa (Figure 1). Routine blood testing showed the following: white blood cells (WBC), 7.5×109/L; hemoglobin (Hg), 122 g/L; neutrophil granulocyte ratio (N%), 70.2%.
Arterial blood gas analysis showed the following: pH, 7.39; PO2, 67 mmHg; PCO2, 50 mmHg. Rheumatism immune test showed the following: antinuclear antibodies (ANA) spectrum, all 3 anti-neutrophil cytoplasmic antibody (ANCA) terms were negative. Procalcitonin (PCT), erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), and TB T-cell spot (TB t-spot) tests were all negative. Pulmonary function test showed the following: severe restrictive ventilatory defect; severe decline in diffuse function; forced vital volume (FVC) 1.61 L, accounting for 41.5% of the expected value; forced expiratory volume in one second (FEV1) 1.33 L, accounting for 41.5% of the expected value; lung diffusing capacity for carbon monoxide single breath (DLCO SB), 2.89 mmol/min/kPa, accounting for 31.7% of the expected value; and no bronchodilator response.
After admission to the hospital, the patient received symptomatic treatment, but no obvious improvement was observed. As the underlying cause of bilateral ILD remained unknown, the attending physician suggested a multidisciplinary discussion. Experts recommended bronchoscopic lung cryobiopsy or surgical lung biopsy (SLB) to achieve a definite diagnosis. The patient decided to undergo a SLB after consideration. Post operation, the patient’s dyspnea was aggravated; meanwhile, the blood leukocytes, CRP, PCT, and other infective indicators increased, so the patient was admitted to Respiratory Intensive Care Unit (RICU) for intensive treatment. Three days later, the patient gradually returned to the preoperative state with enhanced anti-infective drugs, high-flow nasal cannula oxygen therapy, and intensive care.
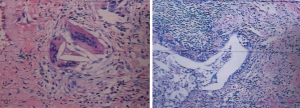
The patient’s lung biopsy pathology revealed alveolar epithelium adenoma—hyperplasia, alveolar cavity macrophage accumulation, interstitial fibrosis, patchy distribution around the bronchioles, lymphocyte infiltration, visible non-necrotizing granulomas and bronchiolitis. All combined characteristics were consistent with chronic hypersensitive pneumonia changes (lower lobe of right lung) (Figure 2).
The clinical diagnoses of the patient are chronic interstitial pneumonia (related to sulfasalazine), pulmonary infections, and ulcerative colitis. The clinical treatment of the patient are as follows: discontinuation of sulfasalazine and oral administration of glucocorticoids; follow-up visit after 3 months.
iMDT discussionOther Section
- Introduction
- Case presentation
- iMDT discussion
- Several issues regarding the diagnosis and treatment of this patient were further discussed as follows
- Conclusions
- Acknowledgments
- Footnote
- References
The patient was admitted due to cough and shortness of breath for more than 4 years, exacerbation for one month without a confirmed diagnosis despite visiting many hospitals. After admission to Xiangya hospital with the extensive and comprehensive investigations, SLB revealed that the lung histology was consistent with chronic hypersensitive pneumonia. However, chronic hypersensitive pneumonia requires an allergen, and it was unclear if the patient’s imaging had the characteristics of chronic hypersensitive pneumonia, and whether the pathological analysis could rule out other considerations.
Department of Radiology
The CT features that best differentiated chronic HP (cHP) were lobular areas with decreased attenuation and vascularity, centrilobular nodules, and absence of lower zone predominance of abnormalities (3). This patient’s lung HRCT showed diffuse ground-glass opacities in both lungs, along with traction bronchiectasis, a small range of honeycomb cysts, and uneven decreased attenuation, the lesions were mainly distributed around the bronchial vascular bundle, which were consistent with the imaging features of cHP.
Department of Pathology
Thoracoscopic lung biopsy was performed on the lower right lung. The main pathological findings included alveolar adenomatous hyperplasia, macrophage accumulation in the alveolar space, interstitial fibrosis, and patchy distribution (especially around the bronchioles), along with lymphocytic infiltration. Non-necrotic granuloma and bronchiolitis were visible. Taking all the imaging features into account, together with relevant clinical findings, we may consider a diagnosis of cHP.
Department of Rheumatology
The patient is a 44-year-old male with mainly respiratory symptoms. He had no symptoms of joint pain, fever, rash, dry mouth, dry eyes, or Raynaud’s phenomenon. Renal function test and routine urine test were normal. The results of serological testing for connective tissue diseases (CTD) were negative. Thus, no evidence supported a diagnosis of CTD, and interstitial pneumonia is considered to be associated with non-CTD.
Department of Pulmonary and Critical Care Medicine
The patient is a middle-aged adult whose cough and dyspnea gradually worsened during the past 4 years. Bilateral CT scans revealed diffuse lesions, mainly ground-glass opacities, mosaic attenuation, and fibrosis. Pulmonary function test showed decreased carbon monoxide diffusion capacity and restrictive ventilatory dysfunction. Thus, an ILD may be considered. However, ILD comprises of a very large group of more than 200 different entities. Although a diagnosis of idiopathic interstitial pneumonia was made in another hospital two years ago, other causes need to be ruled out since the disease was found to be “idiopathic”. One of the common causes is CTD. Since serological tests showed negative results and there were no CTD-related symptoms such as joint pain and rash, the possibility of CTD-related ILD can be ruled out. Since the patient is a civil servant, and he had no history of dust exposure or bird-breeding, the possibility of occupational interstitial pneumonia can also be ruled out. The findings of lung CT and pathology supported a diagnosis of cHP. The patient had a history of ulcerative colitis (involving sigmoid colon and rectum), which had been treated with long-term use of sulfasalazine, a drug that could cause interstitial pneumonia. The duration of sulfasalazine use was consistent with that of interstitial pneumonia. Thus, a diagnosis of cHP (and/or interstitial pneumonia) is highly likely. Since diffuse lesions and fibrosis were noticed in both lungs, drugs that might have caused these problems should be discontinued first, followed by the administration of glucocorticoids and/or anti-fibrotic drugs (e.g., pirfenidone).
Several issues regarding the diagnosis and treatment of this patient were further discussed as followsOther Section
- Introduction
- Case presentation
- iMDT discussion
- Several issues regarding the diagnosis and treatment of this patient were further discussed as follows
- Conclusions
- Acknowledgments
- Footnote
- References
Question 1: Can a diagnosis of chronic hypersensitivity pneumonia be made in this case?
Expert opinion 1: Dr. Martin Kolb & Dr. Amornpun Wongkarnjana
The HRCT findings of diffuse ground-glass opacities with a geographic pattern, peribronchovascular interstitial changes, architectural distortion and mosaic attenuation are suggestive of cHP. In addition, the pathological findings of peribronchiolar lymphocytic infiltration, interstitial fibrosis and non-necrotizing granuloma do support this diagnosis. The question about the cause and potential differential diagnosis is complex. IBD themselves can cause ILD. Among IBD-related ILD, organizing pneumonia and non-specific interstitial pneumonia are most commonly reported, but extensive fibrosis is extremely rare (4,5). Some cases of IBD-related pulmonary granulomatous inflammation have been reported, however being related to Crohn’s disease rather than ulcerative colitis.
Expert opinion 2: Dr. Reoto Takei
The clinical course was an acute-on-chronic type. Though radiological and pathological findings were consistent with cHP, these findings were only in the acute phase and it did not necessarily reflect the findings of the chronic phase. Moreover, the patient had no history of environmental exposure or investigation of bronchoalveolar lavage. Therefore, this case was not diagnosed as cHP.
Expert opinion 3: Dr. Yoshinori Tanino
Radiological and pathological findings are consistent with chronic hypersensitivity pneumonia (cHP). In fact, lung parenchymal diseases are reported to be induced by drugs such as sulfasalazine in IBD. On the other hand, IBD itself can induce lung parenchymal diseases. Although organizing pneumonia is most reported, other ILDs such as non-specific interstitial pneumonia are observed as well. Furthermore, even granuloma has been shown histopathologically. These findings show the difficulty in differentiation between drug toxicity (cHP) and IBD lung parenchymal involvement. cHP is my first tentative diagnosis, however, IBD lung parenchymal involvement and overlap between these two disorders cannot be ruled out.
Question 2: Was the disease caused by sulfasalazine use?
Expert opinion 1: Dr. Martin Kolb & Dr. Amornpun Wongkarnjana
The history is unclear and does not specify whether the patient developed respiratory problems before or after sulfasalazine initiation. Although sulfasalazine is a potential cause of this patient’s ILD regarding his clinical course, eosinophilic pneumonia is most commonly reported in sulfasalazine-related pneumonitis, followed by organizing pneumonia and sarcoid-like granulomatous inflammation. The best approach to make a causal relationship between sulfasalazine and his disease would be to assess the clinical and radiological response after discontinuing sulfasalazine.
Expert opinion 2: Dr. Reoto Takei
Though sulfasalazine was reported to be a cause of ILD, this case had a long history of lung fibrosis. Sulfasalazine is unlikely to be the cause of the disease.
Expert opinion 3: Dr. Yoshinori Tanino
As mentioned above, it is possible that sulfasalazine induced these findings in this case. Although it is reported that symptoms appear after 2–6 months of drug use in most cases, they appear after many years in a few cases. However, again, it is difficult to distinguish drug toxicity from IBD lung parenchymal disease. If clinical and radiological findings are improved after withdrawal of sulfasalazine use, the possibility of cHP by sulfasalazine will increase.
Question 3: Was the patient at high risk for acute exacerbation (AE) during SLB?
Expert opinion 1: Dr. Martin Kolb & Dr. Amornpun Wongkarnjana
To date, there are no well-designed studies identifying risk factors for AE of progressive fibrosing ILD. In idiopathic interstitial pneumonias especially idiopathic pulmonary fibrosis (IPF), forced vital capacity (FVC) less than 50% predicted, DLCO below 40% predicted and requirement of supplemental oxygen at baseline are associated with increased risk of perioperative AE (6). Primarily intrathoracic surgeries increase the risk of AE in IPF patients (AE-IPF). The proposed pathogenesis of surgery-induced AE-IPF include epithelial injury, coagulation abnormalities, autoimmunity, ventilator-induced lung injury, oxygen toxicity and micro-aspiration. Considering these known risk factors, the patient described in this case was indeed at higher risk of developing post-operative AE. This highlights that for every case of lung biopsy, one has to make a thorough risk-benefit assessment.
Expert opinion 2: Dr. Reoto Takei
The clinical course was not chronic and no information on underlying ILD was obtained. Therefore, it is difficult to evaluate whether high risk for AE or not.
Expert opinion 3: Dr. Yoshinori Tanino
Although it is difficult to predict the onset of AE, Sato et al. have retrospectively analyzed 1,022 lung cancer patients with ILDs and identified the risk factors for predicting AE after lung resection (7). They are history of AE, surgical procedures, usual interstitial pneumonia (UIP) appearance in CT scan, male sex, preoperative steroid use, elevated serum KL-6 level, and low vital capacity. Although the subjects included in the study were different, to evaluate these risk factors are useful. The present patient had two risk factors (male sex and low vital capacity). However, these two risk factors are not relatively important from the weight of variables. These results suggest that the patient was not at high risk for AE.
Question 4: If drug treatment is still considered, which drug is preferred? A glucocorticoid or an anti-fibrotic drug?
Expert opinion 1: Dr. Martin Kolb & Dr. Amornpun Wongkarnjana
This patient had two respiratory problems, acute post-operative respiratory deterioration and chronic progressive ILD. Firstly, short-term high-dose corticosteroid therapy should be considered to manage the AE. In our practice, corticosteroid is usually tapered off in 2–4 weeks depending on clinical response. Secondly, regarding his ILD, if the patient still had progressive symptoms after stopping sulfasalazine or could not taper off steroid, we would consider adding azathioprine or mycophenolate. From a retrospective review, adding azathioprine or mycophenolate resulted in successful reduction of steroid without lung function decline and could decrease steroid side effects, although this did not change survival of cHP (8).
Anti-fibrotic agent is an interesting novel therapy for progressive fibrosing ILD. There was a small retrospective study from Japan showing efficacy of pirfenidone to slow FVC decline in cHP patients but no survival benefit (9). The INBUILD trial is a randomized-control study comparing nintedanib to placebo in the treatment of progressive fibrosing ILD (10). The study has recently been published and shows that nintedanib can slow the annual rate of FVC decline in the patients with UIP-like fibrotic pattern. Given that this patient had significant fibrotic changes from both CT scan and histopathology, he could benefit from anti-fibrotic therapy in terms of slowing disease progression.
Expert opinion 2: Dr. Reoto Takei
I prefer to evaluate treatment responsiveness with glucocorticoid.
Expert opinion 3: Dr. Yoshinori Tanino
Corticosteroid therapy is considered to be standard for cHP as well as IBD lung parenchymal diseases, although there are no conclusive results to recommend the therapy. Regarding anti-fibrotic drugs, they may be effective for progressive fibrotic parenchymal diseases, especially with a UIP-like pattern. From this point of view, a glucocorticoid is preferred, however, an anti-fibrotic drug can be considered for the present patient.
Question 5: What is the patient’s prognosis?
Expert opinion 1: Dr. Martin Kolb & Dr. Amornpun Wongkarnjana
Patients with cHP and progressive disease requiring immunosuppressive agents have a significantly poorer prognosis than patients with stable disease who did not receive therapy (8). Post-operative AE could impair his lung function, on the other hand avoiding exposure to sulfasalazine would hopefully prevent further inflammation and progression of the disease.
Expert opinion 2: Dr. Reoto Takei
It is very difficult to predict the prognosis. The responsiveness to glucocorticoid and respiratory conditions were important to predict the prognosis in this case.
Expert opinion 3: Dr. Yoshinori Tanino
It has been reported that the presence of radiological or histological findings of fibrosis suggests worse prognosis in cHP. Recently, Salisbury et al. analyzed the prognosis of patients with HP. They divided the patients into three groups (honeycomb present, non-honeycomb fibrosis present, and no fibrotic) according to HRCT findings, and showed that the subjects with no fibrotic HP had median transplant-free survival >14.7 years, compared with >8.0 years for the fibrotic phenotype and 2.8 years for the honeycomb phenotype (11). These results suggest that the patient’s prognosis is better than the honeycomb phenotype and worse than no fibrotic HP.
ConclusionsOther Section
- Introduction
- Case presentation
- iMDT discussion
- Several issues regarding the diagnosis and treatment of this patient were further discussed as follows
- Conclusions
- Acknowledgments
- Footnote
- References
The diagnosis and differentiation of ILDs is often very challenging. When we consider cHP, we need sufficient evidence to rule out other ILDs. When lobular areas with decreased attenuation and vascularity, centrilobular nodules, and absence of lower zone predominance of abnormalities are found on chest CT imaging, we should not forget the possibility of cHP. Both drug toxicity and IBD itself can induce pulmonary interstitial lesions, and the medical history and pathological examination of the diseased tissue are very important. The best way to establish a causal relationship between a drug and cHP is to evaluate the clinical and radiological response after discontinuation of the drug. Corticosteroid therapy is considered to be standard for cHP, and we need more data from ongoing clinical trials to support benefit of anti-fibrotic therapy on cHP. Different subtypes of cHP have different prognosis, the prognosis of non-fibrotic subtype is better than honeycomb present subtype and non-honeycomb fibrosis present subtype.
AcknowledgmentsOther Section
- Introduction
- Case presentation
- iMDT discussion
- Several issues regarding the diagnosis and treatment of this patient were further discussed as follows
- Conclusions
- Acknowledgments
- Footnote
- References
Funding: None.
FootnoteOther Section
- Introduction
- Case presentation
- iMDT discussion
- Several issues regarding the diagnosis and treatment of this patient were further discussed as follows
- Conclusions
- Acknowledgments
- Footnote
- References
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jxym.2019.12.01). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
ReferencesOther Section
- Introduction
- Case presentation
- iMDT discussion
- Several issues regarding the diagnosis and treatment of this patient were further discussed as follows
- Conclusions
- Acknowledgments
- Footnote
- References
- Selman M. Hypersensitivity pneumonitis. In: Schwarz M, King TE Jr. editors. Interstitial lung disease. 5th edition. Shelton, CT: People’s Medical Publishing House-USA,2011:597-625.
- Selman M, Pardo A, King TE Jr. Hypersensitivity pneumonitis: insights in diagnosis and pathobiology. Am J Respir Crit Care Med 2012;186:314-24. [Crossref] [PubMed]
- Silva CI, Müller NL, Lynch DA, et al. Chronic hypersensitivity pneumonitis: differentiation from idiopathic pulmonary fibrosis and nonspecific interstitial pneumonia by using thin-section CT. Radiology 2008;246:288-97. [Crossref] [PubMed]
- Cozzi D, Moroni C, Addeo G, et al. Radiological Patterns of Lung Involvement in Inflammatory Bowel Disease. Gastroenterol Res Pract 2018;2018:5697846. [Crossref] [PubMed]
- Majewski S, Piotrowski W. Pulmonary manifestations of inflammatory bowel disease. Arch Med Sci 2015;11:1179-88. [Crossref] [PubMed]
- Kolb M, Bondue B, Pesci A, et al. Acute exacerbations of progressive-fibrosing interstitial lung diseases. E Eur Respir Rev 2018;27:180071. [Crossref] [PubMed]
- Sato T, Kondo H, Watanabe A, et al. A simple risk scoring system for predicting acute exacerbation of interstitial pneumonia after pulmonary resection in lung cancer patients. Gen Thorac Cardiovasc Surg 2015;63:164-72. [Crossref] [PubMed]
- Adegunsoye A, Oldham JM, Fernández Pérez ER, et al. Outcomes of immunosuppressive therapy in chronic hypersensitivity pneumonitis. ERJ Open Res 2017;3:00016-2017. [Crossref] [PubMed]
- Shibata S, Furusawa H, Inase N. Pirfenidone in chronic hypersensitivity pneumonitis: a real-life experience. Sarcoidosis Vasc Diffuse Lung Dis 2019;35:139-42.
- Flaherty KR, Wells AU, Cottin V, et al. Nintedanib in Progressive Fibrosing Interstitial Lung Diseases. N Engl J Med 2019;381:1718-27. [Crossref] [PubMed]
- Salisbury ML, Gu T, Murray S, et al. Hypersensitivity Pneumonitis: Radiologic Phenotypes Are Associated With Distinct Survival Time and Pulmonary Function Trajectory. Chest 2019;155:699-711. [Crossref] [PubMed]
Cite this article as: He Y, Xia Y, Hu Y, Zhou H, Zhao H, Luo Q, Ma W, Kolb M, Wongkarnjana A, Takei R, Tanino Y, Meng J. Multidisciplinary team approach on a case of bilateral interstitial pneumonia. J Xiangya Med 2020;5:1.


