
Pneumatic dilation in the management of achalasia
Introduction
Historical considerations
In the late 17th century, Sir Thomas Willis performed one of the earliest successful dilation attempts in an individual with suspected achalasia using a whalebone with a sponge (1). There was no appreciable advancement in technique until the 20th century, when use of a water-filled balloon was reported for the treatment of cardiospasm in 1921—a technique increasingly adopted as the century progressed (1).
Evolution late 20th/21st centuries: re-emergence of endoscopic treatment
By the 1960s, pneumatic dilation (PD) had become an accepted treatment for achalasia, although the exact parameters had yet to be standardized. The aim of PD is to disrupt the lower esophageal sphincter (LES) muscle fibers with radial force (1). A relatively large case series in 1975 showed the enormous potential of PD, with 29 out of 31 patients having improved after PD without complication (2). Bougie dilation for achalasia had also been explored, but this technique rapidly fell out of favor, with a study of patients with Chagas mega-esophagus (with Chagas causing a similar loss of nitrergic inhibition resulting in secondary achalasia) demonstrated very clearly the lack of efficacy of bougie dilation compared to PD (3).
Technique and equipment
Over the years, the technical principles of PD have remained remarkably preserved, though equipment has evolved considerably. A part of this advancement is derived from the standardization of terminology with the Chicago classification (4). The gold standard for diagnosis of achalasia is high resolution manometry, which allows subtyping of disease into achalasia types 1, 2, or 3, with implications for the treatment modality (see below) selected. The critical abnormality detected on manometry is an elevated integrated relaxation pressure of the LES which correlates with impaired lower esophageal relaxation. In order to qualify as achalasia, there must be consistently aberrant peristalsis or even aperistalsis. While generations of physicians have been taught the bird’s beak esophagram abnormality that correlates with achalasia, barium esophagram does not officially make a diagnosis of achalasia. It does, however, define overall esophageal anatomy in addition to serving as a useful adjunct for confirming the diagnosis and establishing an objective baseline for comparison post-dilation (5).
PD performed in a standardized fashion has the highest chance for technical success (6). The most common PD device used today is the Boston Scientific Rigiflex™ balloon system, though other similar products are in use as well. The balloon system consists of a 10-cm long, non-compliant balloon mounted on a flexible catheter with radiopaque markers defining the balloon location. Catheters are available in 30, 35, and 40 mm diameters with most patients’ first dilation starting at 30 mm. During the procedure, endoscopy is used to pass a guidewire into the stomach, over which the balloon catheter is advanced into the esophagus through the oropharynx. Fluoroscopic guidance is used to ensure appropriate positioning, and the device inflated until the “waist” (a narrowing in the balloon under fluoroscopy representing the LES; Figure 1) disappears for between 15–120 seconds (5) (Figure 2). The procedure itself can be repeated every 2–4 weeks in a sequential fashion with increasing balloon size. The patient may be observed for some time after the procedure, with contrast-assisted esophagram recommended post-procedure especially if the patient is experiencing significant pain or fever (1).
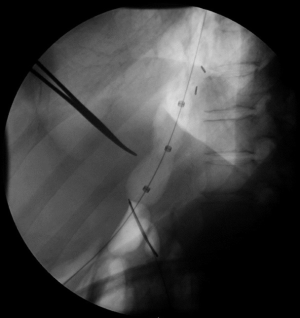
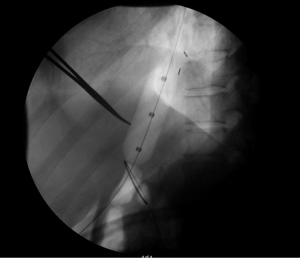
Clinical use of PD
Efficacy
When deciding which patients should undergo PD, clinicians should weigh the likelihood of long-term efficacy (including need for repeat interventions) with the potential for complications. A general rule of thumb is that if the patient is thought to not be clinically well enough to undergo a surgical myotomy, PD should not be considered due to what would likely be a risker surgical intervention in the emergency setting (although PD can be considered in high volume centers with appropriate availability of surgical back-up) (7). That said, our group has offered PD to appropriately-counselled patients for whom surgery is not offered yet whose lifespan is expected to outlast the limited effects of Botox injections.
PD efficacy was initially determined using relatively simplistic measurements of treatment effect such as improvement of esophageal emptying or ability to resume per os intake with improved symptoms (2). Only by the late 1980s and early 1990s, approximately 20 years after the introduction of PD, did long-term efficacy data first emerge. With a median follow-up of 4 years, between 61% and 76% of patients who underwent PD as their first intervention had good or excellent responses with more sophisticated measures used to determine success or complication including not only esophagram but also pH testing and esophageal manometry (8). In a cohort of 54 patients examined prospectively over a 10-year period, diameter of the balloon dilation had some predictive value for treatment efficacy, with younger patients fairing less well (9). While these studies were being published, various management algorithms were introduced, including graded PD (starting at a diameter typically of 30 mm but then increasing in size depending on whether or not the first treatment was deemed a technical success) (6), which was estimated to have an efficacy of 93% (10). Since then, additional factors for poor response have been suggested including male gender, esophageal diameter less than 3 cm, lower esophageal pressure greater than 30 mmHg, as well as esophageal body pressure greater than 15 mmHg (11). Generally, type 1 and type 3 achalasia respond less favorably to PD compared to type 2 achalasia (12).
Of late, however, the long-term effectiveness of PD began to be questioned. In one study, the estimated efficacy of only 50% with a median number of 4 dilations (13) with another showing that only half of patients continued to be in clinical remission 15 years after initial PD (14). It is important to note, though, that the use of PD for achalasia has generally remained a first-line treatment, with later studies this decade suggesting once again that in the appropriate context upwards of >90% of patients can achieve good outcomes with PD, which remain durable.
Complications
Complications of PD have been described since the modern iterations of this treatment, and can be considered as mainly falling into two categories: peri-procedural (hours to days after dilation) and long term (months to years). Initial experience described relatively minor complications including bradycardia, ECG changes (15), and mucosal tears after successful dilation (16). Leak was also described post-dilation, although it was recognized that it could be managed conservatively (2). Gastric perforation was also an early reported complication (17). As PD technique matured over the course of the 1970s and 1980s, additional complications were reported including delayed esophageal perforation (18) and transient esophageal obstruction (19).
Perforation is the most feared immediate complication of PD with esophageal perforation occurring in 4% of patients in the landmark European Achalasia trial described in detail later in this manuscript. Perforation occurred in 4/95 patients treated initially with the 30 mm PD (3 with initial dilation to 30 mm and 1 with second dilation to 35 mm). Perforation was more likely to occur in those who were older at the time of PD. When the 35 mm was used initially, the perforation rate jumped to 31%. None of these patients required surgical repair. Many commentators and subsequent studies have suggested that a 4% perforation rate is likely on the high end of clinical practice. In a large series there was an estimated complication rate of 9% including perforation, hematemesis, fever, and angina, with a perforation rate of only 1.7% (20). Use of elevated inflation pressures and prior dilation were found to increase the risk of developing a complication. Most recently, a large esophageal center in the US reported a very low perforation rate of 0.37% (1/272) over 12 years of PD experience, which they attributed to PD technique and volume of procedures compared to other centers (21).
Given concern for potential perforation, we always obtain post-procedure contrast studies before the patient is discharged from our hospital, but this is not universal practice. In one series, symptom-driven use of post-dilation contrast studies proved sufficient in identifying those patients who would need surgical intervention for perforations (22) and some perforations may be recognized by direct endoscopic visualization (23). If a small perforation is recognized, it can often be managed by clipping alone (24) with surgery recommended for large perforations. Overall, smaller perforations and deep tears respond well to antimicrobial therapy and prolonged NPO status (including total parenteral nutrition) for days to weeks (1). Larger perforations generally would need to be managed by surgical intervention, at which time a laparoscopic Heller myotomy (LHM) can be performed; consideration can also be given to the use of endoscopic stenting in such a scenario (25).
Longer term complications of PD were first published in the 1990s, partially driven both by the advent of botulinum toxin injection as another minimally invasive intervention as well increasingly sophisticated, minimally-invasive surgical approaches (26). Some in the field felt that important chronic adverse events of PD were being ignored. These included delayed recognition of perforation, development of intramural hematomas, diverticula at the gastric cardia, prolonged chest pain, and a need for eventual surgical intervention of upwards of a third of patients (27). It was estimated that the amount of reflux after PD was similar to myotomy without fundoplication suggesting a major disadvantage of dilation compared to surgical myotomy when an anti-reflux component can be added to the procedure (28). The consensus, though, given (I) an estimated effective treatment rate of 65–90%; (II) minimal reflux; (III) and efficacy/safety of emergent surgery for perforation being similar to primary surgical intervention settled on PD remaining a reasonable technique for managing achalasia (26). By the 2000s, though, it was clearer that younger patients may benefit from primary surgical intervention compared to PD (29).
Failures
When deciding that PD has failed, various factors need to be considered. Generally, PD is deemed a failure if (I) subjectively there is a recurrence of symptoms that prompted evaluation for achalasia or (II) objectively by a measure of impaired esophageal function such as timed barium esophagram or functional luminal imaging probe (EndoFLIP). A timed barium esophagram is a technique in which a standardized height of barium in the esophageal body is measured in the upright position, with emptying measured at specific intervals of time (such as 1 or 5 minutes). Later studies using timed barium esophagram after dilation demonstrated that patients who report symptom improvement can continue to have poor esophageal emptying (30). Additionally, upwards of 30% of patients who report immediate symptomatic improvement after PD will have ongoing, impaired esophageal emptying when assessed by timed barium swallow. In this group, 90% will eventually be deemed treatment failures with longer-term follow up (31). EndoFLIP is a newer technology which uses an endoscopically-placed, fluid-filled balloon to assess the distensibility of the LES using technology called impedance planimetry. LES distensibility is impaired in achalasia, and early data suggests that ongoing impaired distensibility after an intervention (such as PD) predicts need for further therapy (32). Failure may not be purely motor, though, but actually sensory. As early as 1975, in the previously mentioned case series, one patient who failed to improve admitted that her dysphagia symptoms flared during her “serious domestic problems”, suggesting modulation of dysphagia complaints via a brain-gut mechanism that was not necessarily related to a primary motor abnormality itself (2).
Our practice is to perform a timed barium esophagram and/or repeat manometry when a patient complains of post-PD dysphagia to see if complaints correlate with delayed esophageal emptying or impaired LES relaxation. Endoscopy can be considered depending on the length of time the patient has had symptoms that eventually received a diagnosis of achalasia given the concern that achalasia can predispose to esophageal cancer (33). As part of the graded PD technique described above, dilation is repeated with increasing balloon size up to 40 mm at which point myotomy can be considered if there is an absence of symptom improvement. Before embarking on repeat dilation or myotomy, medical management of symptoms (such as acid suppression or neuromodulation with agents such as tricyclic antidepressants) can be considered. After each dilation, recurrence of symptoms prompts a repeat timed barium esophagram or EndoFLIP assessment to determine if residual symptoms are mediated by ongoing failure of LES relaxation or may be reflective of worsening esophageal body peristalsis, which is not improved with any of our current achalasia interventions.
PD in children
In that achalasia can appear early in life, PD can be performed in pediatric patients as well. However, the literature is limited in quality due to low case volume and the relative rarity of achalasia in children compared to adults. One small case series in 1981 involving 10 patients between 10 and 17 years of age showed good response in 8 of the 10, with improved vomiting, dysphagia, and resumption of weight gain, with fever and chest pain being seen in 3 out of 18 procedures (34). More recent studies have demonstrated durable symptomatic improvement in children (35,36). However, based on experience from young adult patients, with less favorable long-term outcomes and increased need for additional intervention, there has been less enthusiasm for PD in children overall, and it is thought to be less effective than Heller myotomy (37).
PD versus primary Heller myotomy
Many small studies have compared LHM and PD for the treatment of achalasia, focusing on parameters such as symptomatic improvement, retreatment rates, and quality of life. Despite the available evidence, the optimal treatment strategy remains controversial (38,39). In a landmark European, randomized controlled trial (RCT) from 2011 including 201 patients with idiopathic achalasia, therapeutic success as measured by Eckardt score (including symptoms such as dysphagia, retrosternal pain, and regurgitation) was found to be equivalent between LHM and PD (40). LHM with Dor fundoplication had success rates of 93% at 1 year and 90% at 2 years, while PD was successful in 90% at 1 year and 86% at 2 years (P=0.46). LES pressure, esophageal emptying, and quality of life were also similar between groups. LHM was complicated by mucosal tears in 12% of patients, while PD was complicated by esophageal perforation in 4% of patients. Five-year data from this trial revealed success rates of 84% for LHM and 82% for PD (41). Retreatment with additional dilations was ultimately required in 25% of the PD patients. Though retreatment was not allowed for LHM patients in this trial, a small study has shown that PD can be done safely in patients with failed LHM (42). A long-term analysis of the same trial (at least 5 years of follow-up), showed that PD and LHM have comparable success rates, but 25% of PD patients required re-dilation during follow-up (41).
A similar RCT based in Canada, compared Achalasia Severity Questionnaire (ASQ) scores among 50 patients randomized to LHM or PD and found statistically-similar score improvements at 1 and 5 years (43). There were also no differences in quality of life or esophageal physiology measures between the two treatments. Two patients developed post-LHM surgical site infections and one LHM patient had an esophageal injury that was repaired during the procedure. There were no serious complications in patients who underwent PD. No patients who underwent LHM required retreatment, compared with five patients who underwent PD.
The LHM vs. PD controversy has been further evaluated in two comprehensive meta-analyses, each including 5 RCTs, though there is significant overlap in the included studies (44,45). Baniya et al. found that LHM was associated with improved response rates at 3 months (OR 0.52, 95% CI: 0.32, 0.85) and 1 year (OR 0.47, 95% CI: 0.22, 0.99). Response rates were also improved for LHM at 5 years, though this was not statistically significant and the confidence intervals were wide (OR 0.44, 95% CI: 0.08, 2.39). While the authors concluded that these data suggest similar rates of success at 5 years between the two methods, additional long-term follow up is needed for a definitive comparison, especially given the apparent advantage of LHM in the short-term. The second meta-analysis by Cheng et al. corroborated these findings.
While primary outcomes have generally focused on symptomatic improvement of dysphagia, many other factors play a role in individual therapy choices. PD can be performed in the outpatient setting without general anesthesia, but unintended perforations can require complex surgical intervention. On the other hand, LHM requires general anesthesia and abdominal wall incisions, but perforations are more easily repaired intraoperatively. Post-procedure rates of gastroesophageal reflux in PD vs. LHM are not well established. While one large study showed similar rates of GERD by pH monitoring (15% in PD group vs. 23% in LHM group, P=0.28) (40), a smaller study showed 27.7% of patients after PD had significant reflux, compared to 4.7% of LHM patients (P=0.003) (46). In both studies, partial fundoplication was performed. The availability, safety, and operator skill of either treatment in a given region would also weigh heavily in the decision-making process.
Many of these initial studies comparing PD to LHM have lacked information on achalasia sub type, only defined since the widespread adoption of the Chicago classification of esophageal motility. The majority of studies, including the European achalasia trial, have not yet considered the type of achalasia as a predictive factor in rates of success. Preliminary data has suggested differential treatment response based on the type of achalasia, but trials have not yet been designed to fully investigate these outcomes using achalasia subtyping (47). In a post-hoc analysis of the European achalasia trial, type 2 achalasia patients had significantly more improvement with PD compared to LHM, while the converse was true for type 3 patients. In all cases, type 2 achalasia responds more favorably to both treatments in comparison to types 1 or 3, but it is not yet known whether the optimal treatment strategy may depend on the type of achalasia.
PD versus other approaches
PD after failed myotomy
As the first systematic reviews of the use of PD were being published in the 1970s, so were the first reports of the use of PD after failed myotomy (48). An initial comparison of long-term efficacy found that patients who underwent dilation after a failed myotomy improved as much (if not more) compared to patients who underwent a primary treatment (8). As LHM became the favored surgical approach for achalasia, inevitably a subset of patients failed to respond, which begged a natural question: can PD be used as a rescue therapy for failed myotomy? It was initially hypothesized that a failed myotomy (for example, those with recurrent dysphagia) was due to either an incomplete myotomy or fibrosis at the distal site of the myotomy, with the belief that such etiologies would respond well to PD (49). The principal fear early on was whether or not PD after LHM would lead to an unacceptably high risk of perforation. In one crucial study of 22 patients, however, it was shown that PD after a failed Heller myotomy resulted in no perforations, suggesting the safety of dilation after myotomy (50). Further studies have continued to emphasize not only the relative safety of PD after myotomy but also the efficacy and durability of the treatment effect, albeit with relatively small sample sizes (51).
PD vs. per-oral endoscopic myotomy (POEM)
The newest arrival to the achalasia treatment landscape is the POEM. As discussed later in this review series, the initial POEM data is promising. Unfortunately, most early investigations have compared POEM to surgical myotomy (LHM), not PD (1). Such comparisons, given their basis in endoscopic management, would be clinically useful. Of the limited data that does exist, early indications suggest that POEM may be more effective than PD. A small RCT compared PD versus POEM and found that three months after treatment, success was achieved in 63/64 (98.4%) patients in the POEM group versus 52/66 (78.8%) patients in the PD group, P<0.01; similar success of POEM versus PD was noted 1 year after treatment. However, reflux was noted to be more problematic after POEM compared to PD (52). A similarly sized retrospective review suggested that while short term symptom improvement in patients treated with both PD and POEM is similar, the treatment effect is more durable for POEM (especially for type 3 achalasia patients) (53). That said, another study with a similar sample size found that both PD and POEM led to improved clinical outcomes without significantly different responses between therapy modalities (54). POEM has also been suggested as a rescue treatment in patients who fail PD, with similar rates of success compared to those patients with achalasia who have not undergone prior PD (although the procedure tends to be more technically difficult after PD) (55).
PD vs. dilation guided by impedance planimetry
There is an increasingly recognized concept of a “treatment” gap in the intersection between surgical and endoscopic management of achalasia: those patients with symptoms severe enough so that botulinum injection is not expected to work, but who cannot undergo PD, LHM, or POEM due to comorbidities. This can be especially problematic among elderly patients, who are known to suffer a higher rate of complications after PD (1). Impedance planimetry, which can be used to measure distensibility, cross sectional area, and diameter can be employed with dilation capability in the form of EsoFLIP (32,56). Impedance planimetry can measure real time LES distensibility to simultaneously minimize the dilation size (to reduce the chance of perforation) but also ensure clinically-significant treatment response. Initial animal studies are promising (57); unfortunately, limited to no systematic clinical evidence exists at the current time for use of EsoFLIP (let alone comparison to PD).
Conclusions
The past 50 years have seen significant technologic advances in terms of the endoscopic management of achalasia. The most recent international guidelines best place PD in the appropriate modern context (58)(59), recognizing that PD is the most effective non-surgical option for the management of achalasia. Factors favoring successful PD include older age (>45 years), female gender, narrow esophagus, and type 2 pattern of achalasia. While PD is known to be effective, its place in the treatment algorithm for achalasia is still controversial—especially with data on the role of POEM still forthcoming. For many clinicians and their patients, the decision to pursue PD over other treatment options for achalasia is highly personal and relies on an individual collection of physiologic, safety, and patient preference factors.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (David W. Rattner, Ozanan Meireles) for the series “Update on the Diagnosis and Treatment of Achalasia” published in Journal of Xiangya Medicine. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jxym.2019.04.03). The series “Update on the Diagnosis and Treatment of Achalasia” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Markar S, Zaninotto G. Endoscopic Pneumatic Dilation for Esophageal Achalasia. Am Surg 2018;84:473-6. [PubMed]
- Cohen NN. An end point for pneumatic dilation of achalasia. Gastrointest Endosc 1975;22:29. [Crossref] [PubMed]
- Raizman RE, De Rezende JM, Neva FA. A clinical trial with pre- and post-treatment manometry comparing pneumatic dilation with bouginage for treatment of Chagas' megaesophagus. Am J Gastroenterol 1980;74:405-9. [PubMed]
- Kahrilas PJ, Bredenoord AJ, Fox M, et al. The Chicago Classification of esophageal motility disorders, v3.0. Neurogastroenterol Motil 2015;27:160-74. [Crossref] [PubMed]
- Blonski W, Kumar A, Feldman J, et al. Timed Barium Swallow: Diagnostic Role and Predictive Value in Untreated Achalasia, Esophagogastric Junction Outflow Obstruction, and Non-Achalasia Dysphagia. Am J Gastroenterol 2018;113:196-203. [Crossref] [PubMed]
- Jacobs J, Richter JE. Opening the Bird's Beak: Tips and Tricks for Effective Pneumatic Dilation for Achalasia. Am J Gastroenterol 2016;111:157-8. [Crossref] [PubMed]
- Richter JE, Boeckxstaens GE. Management of achalasia: surgery or pneumatic dilation. Gut 2011;60:869-76. [Crossref] [PubMed]
- Cusumano A, Bonavina L, Norberto L, et al. Early and long-term results of pneumatic dilation in the treatment of oesophageal achalasia. Surg Endosc 1991;5:9-10. [Crossref] [PubMed]
- Eckardt VF, Aignherr C, Bernhard G. Predictors of outcome in patients with achalasia treated by pneumatic dilation. Gastroenterology 1992;103:1732-8. [Crossref] [PubMed]
- Kadakia SC, Wong RK. Graded pneumatic dilation using Rigiflex achalasia dilators in patients with primary esophageal achalasia. Am J Gastroenterol 1993;88:34-8. [PubMed]
- Ponce J, Garrigues V, Pertejo V, et al. Individual prediction of response to pneumatic dilation in patients with achalasia. Dig Dis Sci 1996;41:2135-41. [Crossref] [PubMed]
- Patel DA, Lappas BM, Vaezi MF. An Overview of Achalasia and Its Subtypes. Gastroenterol Hepatol (N Y) 2017;13:411-21. [PubMed]
- West RL, Hirsch DP, Bartelsman JF, et al. Long-term results of pneumatic dilation in achalasia followed for more than 5 years. Am J Gastroenterol 2002;97:1346-51. [Crossref] [PubMed]
- Karamanolis G, Sgouros S, Karatzias G, et al. Long-term outcome of pneumatic dilation in the treatment of achalasia. Am J Gastroenterol 2005;100:270-4. [Crossref] [PubMed]
- Mackowiak R, Cohen NN. Cardiovascular effects of pneumatic dilation of the cardio-esophageal junction in achalasia. Gastrointest Endosc 1972;19:15-6. [Crossref] [PubMed]
- Brady PG, Milligan FD. Appearance of the distal esophagus following pneumatic dilation. Gastrointest Endosc 1974;21:30-1. [Crossref] [PubMed]
- Brown FC, Johnson RB, Castell DO. Gastric perforation during pneumatic dilation for achalasia. Gastroenterology 1977;72:983-4. [PubMed]
- Fiorenza V, Messmer JM, Zfass AM. Delayed esophageal perforation following pneumatic dilation for achalasia. Gastrointest Endosc 1988;34:365-6. [Crossref] [PubMed]
- Luppescu NE, Cohen LB. Transient esophageal obstruction after pneumatic dilation for achalasia. J Clin Gastroenterol 1989;11:64-7. [Crossref] [PubMed]
- Nair LA, Reynolds JC, Parkman HP, et al. Complications during pneumatic dilation for achalasia or diffuse esophageal spasm. Analysis of risk factors, early clinical characteristics, and outcome. Dig Dis Sci 1993;38:1893-904. [Crossref] [PubMed]
- Lynch KL, Pandolfino JE, Howden CW, et al. Major complications of pneumatic dilation and Heller myotomy for achalasia: single-center experience and systematic review of the literature. Am J Gastroenterol 2012;107:1817-25. [Crossref] [PubMed]
- Ciarolla DA, Traube M. Achalasia. Short-term clinical monitoring after pneumatic dilation. Dig Dis Sci 1993;38:1905-8. [Crossref] [PubMed]
- Lambroza A, Schuman RW. Pneumatic dilation for achalasia without fluoroscopic guidance: safety and efficacy. Am J Gastroenterol 1995;90:1226-9. [PubMed]
- Wewalka FW, Clodi PH, Haidinger D. Endoscopic clipping of esophageal perforation after pneumatic dilation for achalasia. Endoscopy 1995;27:608-11. [Crossref] [PubMed]
- Elhanafi S, Othman M, Sunny J, et al. Esophageal perforation post pneumatic dilatation for achalasia managed by esophageal stenting. Am J Case Rep 2013;14:532-5. [Crossref] [PubMed]
- Khandelwal M, Ouyang A. Pneumatic dilation for achalasia: are all complications revealed? Gastrointest Endosc 1997;45:437-9. [Crossref] [PubMed]
- Eckardt VF, Kanzler G, Westermeier T. Complications and their impact after pneumatic dilation for achalasia: prospective long-term follow-up study. Gastrointest Endosc 1997;45:349-53. [Crossref] [PubMed]
- Shoenut JP, Duerksen D, Yaffe CS. A prospective assessment of gastroesophageal reflux before and after treatment of achalasia patients: pneumatic dilation versus transthoracic limited myotomy. Am J Gastroenterol 1997;92:1109-12. [PubMed]
- Gockel I, Junginger T, Bernhard G, et al. Heller myotomy for failed pneumatic dilation in achalasia: how effective is it? Ann Surg 2004;239:371-7. [Crossref] [PubMed]
- Vaezi MF, Baker ME, Richter JE. Assessment of esophageal emptying post-pneumatic dilation: use of the timed barium esophagram. Am J Gastroenterol 1999;94:1802-7. [Crossref] [PubMed]
- Vaezi MF, Baker ME, Achkar E, et al. Timed barium oesophagram: better predictor of long term success after pneumatic dilation in achalasia than symptom assessment. Gut 2002;50:765-70. [Crossref] [PubMed]
- Hirano I, Pandolfino JE, Boeckxstaens GE. Functional Lumen Imaging Probe for the Management of Esophageal Disorders: Expert Review From the Clinical Practice Updates Committee of the AGA Institute. Clin Gastroenterol Hepatol 2017;15:325-34. [Crossref] [PubMed]
- Leeuwenburgh I, Scholten P, Alderliesten J, et al. Long-term esophageal cancer risk in patients with primary achalasia: a prospective study. Am J Gastroenterol 2010;105:2144-9. [Crossref] [PubMed]
- Boyle JT, Cohen S, Watkins JB. Successful treatment of achalasia in childhood by pneumatic dilatation. J Pediatr 1981;99:35-40. [Crossref] [PubMed]
- Babu R, Grier D, Cusick E, et al. Pneumatic dilatation for childhood achalasia. Pediatr Surg Int 2001;17:505-7. [Crossref] [PubMed]
- Khan AA, Shah SW, Alam A, et al. Efficacy of Rigiflex balloon dilatation in 12 children with achalasia: a 6-month prospective study showing weight gain and symptomatic improvement. Dis Esophagus 2002;15:167-70. [Crossref] [PubMed]
- van Lennep M, van Wijk MP, Omari TIM, et al. Clinical management of pediatric achalasia. Expert Rev Gastroenterol Hepatol 2018;12:391-404. [Crossref] [PubMed]
- Richter JE. Achalasia - an update. J Neurogastroenterol Motil 2010;16:232-42. [Crossref] [PubMed]
- Lake JM, Wong RK. Review article: the management of achalasia - a comparison of different treatment modalities. Aliment Pharmacol Ther 2006;24:909-18. [Crossref] [PubMed]
- Boeckxstaens GE, Annese V, des Varannes SB, et al. Pneumatic dilation versus laparoscopic Heller's myotomy for idiopathic achalasia. N Engl J Med 2011;364:1807-16. [Crossref] [PubMed]
- Moonen A, Annese V, Belmans A, et al. Long-term results of the European achalasia trial: a multicentre randomised controlled trial comparing pneumatic dilation versus laparoscopic Heller myotomy. Gut 2016;65:732-9. [Crossref] [PubMed]
- Amani M, Fazlollahi N, Shirani S, et al. Assessment of Pneumatic Balloon Dilation in Patients with Symptomatic Relapse after Failed Heller Myotomy: A Single Center Experience. Middle East J Dig Dis 2016;8:57-62. [Crossref] [PubMed]
- Chrystoja CC, Darling GE, Diamant NE, et al. Achalasia-Specific Quality of Life After Pneumatic Dilation or Laparoscopic Heller Myotomy With Partial Fundoplication: A Multicenter, Randomized Clinical Trial. Am J Gastroenterol 2016;111:1536-45. [Crossref] [PubMed]
- Cheng JW, Li Y, Xing WQ, et al. Laparoscopic Heller myotomy is not superior to pneumatic dilation in the management of primary achalasia: Conclusions of a systematic review and meta-analysis of randomized controlled trials. Medicine (Baltimore) 2017;96:e5525. [Crossref] [PubMed]
- Baniya R, Upadhaya S, Khan J, et al. Laparoscopic esophageal myotomy versus pneumatic dilation in the treatment of idiopathic achalasia: a meta-analysis of randomized controlled trials. Clin Exp Gastroenterol 2017;10:241-8. [Crossref] [PubMed]
- Borges AA, Lemme EM, Abrahao LJ Jr, et al. Pneumatic dilation versus laparoscopic Heller myotomy for the treatment of achalasia: variables related to a good response. Dis Esophagus 2014;27:18-23. [Crossref] [PubMed]
- Rohof WO, Salvador R, Annese V, et al. Outcomes of treatment for achalasia depend on manometric subtype. Gastroenterology 2013;144:718-25; quiz e13-4.
- Gelfand MD, Christie DL. Pneumatic dilation under general anesthesia after unsuccessful cardiomyotomy for achalasia. J Clin Gastroenterol 1979;1:317-9. [Crossref] [PubMed]
- Zaninotto G, Costantini M, Portale G, et al. Etiology, diagnosis, and treatment of failures after laparoscopic Heller myotomy for achalasia. Ann Surg 2002;235:186-92. [Crossref] [PubMed]
- Guardino JM, Vela MF, Connor JT, et al. Pneumatic dilation for the treatment of achalasia in untreated patients and patients with failed Heller myotomy. J Clin Gastroenterol 2004;38:855-60. [Crossref] [PubMed]
- Legros L, Ropert A, Brochard C, et al. Long-term results of pneumatic dilatation for relapsing symptoms of achalasia after Heller myotomy. Neurogastroenterol Motil 2014;26:1248-55. [Crossref] [PubMed]
- Ponds FA, Fockens P, Neuhaus H, et al. Peroral Endoscopic Myotomy (POEM) Versus Pneumatic Dilatation in Therapy-Naive Patients with Achalasia: Results of a Randomized Controlled Trial. Gastroenterology 2017;152:S139. [Crossref]
- Ponds FA, Fockens P, Neuhaus H, et al. Peroral Endoscopic Myotomy (POEM) Versus Pneumatic Dilatation in Therapy-Naive Patients with Achalasia: Results of a Randomized Controlled Trial. Gastroenterology 2017;152:S139. [Crossref]
- Sun X, Wang Z, Jiang Q, et al. Short-Term Outcome of Per Oral Endoscopic Myotomy Versus Endoscopic Pneumatic Dilation. Gastroenterology 2017;152:S696-7. [Crossref]
- Ling T, Guo H, Zou X. Effect of peroral endoscopic myotomy in achalasia patients with failure of prior pneumatic dilation: a prospective case-control study. J Gastroenterol Hepatol 2014;29:1609-13. [Crossref] [PubMed]
-
EsoFLIP Dilation - O'Dea J, Siersema PD. Esophageal dilation with integrated balloon imaging: initial evaluation in a porcine model. Therap Adv Gastroenterol 2013;6:109-14. [Crossref] [PubMed]
- Zaninotto G, Bennett C, Boeckxstaens G, et al. The 2018 ISDE achalasia guidelines. Dis Esophagus 2018; [Crossref] [PubMed]
- Vaezi MF, Pandolfino JE, Vela MF. ACG clinical guideline: diagnosis and management of achalasia. Am J Gastroenterol 2013;108:1238-49; quiz 1250. [Crossref] [PubMed]
Cite this article as: Vélez C, Flanagan R, Staller K. Pneumatic dilation in the management of achalasia. J Xiangya Med 2019;4:24.


