Cruciferous vegetables consumption and lung cancer prevention: epidemiological studies and molecular mechanisms
Introduction
The increasing number of people living with cancer and the resulting millions of deaths each year makes it a growing global epidemic (1,2). Lung cancer (LC) is the most commonly diagnosed cancer, and the leading cause of cancer death worldwide (3). The etiology of LC is mainly related to harmful chemicals found in cigarette smoke (CS), such as polycyclic aromatic hydrocarbons (PHAs) and nicotine-derived nitrosamine ketone (NNK) (4). However, growing epidemiological evidences suggest rather consistently that general ambient air pollution may be responsible for increased rates of LC (5-10). According to The International Agency for Research on Cancer (IARC) the carcinogenicity of outdoor air pollution as a complex mixture of particulates [particulate matter (PM)] seems to be consistent with the increased risk of LC in epidemiological research studies and in experimental animals (8,9). LC can also be the result of an endogenous metabolic process, such as in the case of estrogen [17β-estradiol (E2)] and estrogen receptors (ERs) alteration by CS or chemicals with estrogenic activity (6,11). Exposure to environmental pollutants is also associated with changes in the expression of genes involved in DNA damage and repair, inflammation, immune and oxidative stress response, as well as altered telomere length and epigenetic effects such as DNA methylation (12,13). During the initiation stage of carcinogenesis, chemical and physical agents bind and form adducts with DNA. This damage can then be converted into mutations by error-prone repair, which in turn may cause inactivation of tumor suppressor genes or activation of proto-oncogenes and initiate the carcinogenic process (14). One of the most typical DNA adducts is 8-hydroxy-2'-deoxyguanosine (8-OHdG) which is an oxidative product of damage done to the guanine (G) nucleobase (15) that has lower oxidation potential and most easily oxidized among the four nucleobases (16). Several cellular mechanisms repair DNA damage and thereby help to prevent cancer (17), including the base excision repair (BER) system used to repair oxidative lesions such as 8-OHdG (18). However, modern life constantly exposes us to many stressors that lead to the development of chronic illnesses like cancer, and therefore the importance of disease prevention is gaining increasing recognition. In recent years, epidemiological studies have shown that daily fruit and vegetable consumption confers effective protection against various types of cancer, and the preventive effects of their dietary phytochemicals are also well documented (19,20). Among them, the cruciferous (also known as brassica) vegetables have been extensively studied and are especially known for their cancer chemopreventive function (21,22). The cruciferous family, whose name is derived from the cross-shaped flowers, includes several vegetable members with different edible parts, such as; seeds (mustard), flowers (broccoli, cauliflower), leaves (cabbage, brussels sprouts, kale, rocket, watercress), stem (kohlrabi) and roots (radish, turnip). Here, we summarize recent published information about cruciferous vegetables, and describe epidemiological and molecular studies showing their LC prevention properties.
Cruciferous vegetables and LC prevalence: epidemiological studies
Several studies have previously shown an inverse association between cruciferous vegetables consumption and the prevalence of different types of cancer (21-23). The study of Bosetti et al. have analyzed data from a series of case–control studies conducted in Italy and Switzerland between the years 1991 and 2009, that included 11,493 controls and 12,469 cases of 12 different cancer types (24). Using a 78-item food frequency questionnaire (FFQ), which included a specific question on weekly consumption of cruciferous vegetables, the investigators could evaluate the consumption of vegetables at least once a week as compared with no/occasional consumption. The results found a significant reduced risk for developing cancers of the digestive track (oral cavity and pharynx, esophagus, colorectum), breast and kidney, for people who consumed at least one portion (~125 grams) of cruciferous weekly. Moreover, although not statistically significant, the odds ratio (OR) of all the other investigated cancer types (stomach, liver, pancreas, larynx, endometrium, ovary, prostate) were below unity (24). Similar results were also shown in a large number of epidemiological studies that investigated the association of cruciferous vegetable consumption with LC. In the meta-analysis of 31 observational studies, cruciferous vegetable intake was shown to be inversely associated with LC risk (25). A systematic review, which included 19 studies, indicated that cruciferous vegetable intake is inversely associated with LC risk, and that the strongest inverse association was among individuals with homozygous deletion for glutathione S-transferase M1 (GSTM1) and T1 (GSTT1) genotypes (26). Interestingly, this kind of polymorphism seems to be important since individuals with GSTM1/GSTT1 null genotypes metabolize compounds produced by cruciferous vegetables less efficiently, therefore permitting them to remain biologically active for a longer period (27,28). A recent large-scale population-based prospective study found a similar inverse association of cruciferous vegetables intake and LC among current nonsmokers in Japan (29). The study used a 5-years follow-up survey for 82,330 participants who responded to a 138-item FFQ that contained cruciferous vegetables and also included information on smoking. The results found that cruciferous vegetable intake was significantly inversely associated with LC risk among both never and past smokers, however, no such association was observed in current smokers (29). A previous case–control study by Tang et al. using 948 primary LC cases and 1,743 control cases, investigated the association between cruciferous vegetables intake and LC risk among smokers (30). A 44-item FFQ was used to assess usual diet in the years before diagnosis, including raw and cooked cruciferous vegetables, and also requested detailed information on cigarette smoking status. Conversely, the results of this study showed a significant inverse correlation between cruciferous vegetables intake and the risk of developing LC, among smokers, indicating that when more cruciferous vegetables are consumed the resulted OR is lower (30). For cooked cruciferous, the lowest OR (0.59) was for the consumption of >25 servings (1 serving =0.5 cup) per month, while for raw cruciferous, the lowest OR (0.58) was for the consumption of >10 servings per month. Therefore, intake of raw cruciferous vegetables was more strongly inversely associated with LC risk (30).
Phytochemicals of cruciferous vegetables and LC prevention: molecular mechanisms
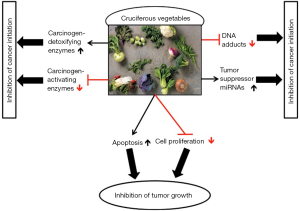
The plant enzyme myrosinase that forms biologically active compounds hydrolyzes glucosinolates, the sulfur-containing compounds that are responsible for the spicy taste and pungent aromas of cruciferous vegetables (23). The degradation products of glucosinolates precursors include; indoles, such as indole-3-carbinol (I3C), and isothiocyanate (ITC), such as phenethyl isothiocyanates (PEITC), allyl isothiocyanate (AITC), benzyl isothiocyanate (BITC), and sulforaphane (SFN), which are some of the most studied components of cruciferous vegetables, and proved to possess anti-cancer properties (23). Numerous studies have revealed the contribution of ITC for various cellular processes used against carcinogenesis, such as inhibition of cell cycle progression and proliferation, and induction of apoptosis (31) (Figure 1). It has been recently suggested that ITC effects against cancer are also related with the activity of nuclear factor-kappaB (NF-κB) transcription factor, which is an active player in human cancers (32). PEITC was shown to be most effective agent in inhibition of CS-related cytogenetic damage, transcriptome alterations, and lung tumorigenesis (33-36). Furthermore, PEITC was shown to significantly inhibit the formation of the xenoestrogen bisphenol A (BPA)-induced DNA adducts (37), and SFN-induced protective phase II enzymes activity, resulted in reduction of E2-induced DNA damage (38). In addition, I3C and its condensation product 3,3’-diindolylmethane (DIM) exhibited potent antitumor activities in a wide range of human cancer cells, including LC (39,40), and SFN was shown to suppress LC through an epigenetic effect (41). Another suggested mechanism for the SFN anti-cancer effects is by modulating microRNA (miRNA) expression (42,43). MiRNAs are endogenous small RNA molecules with diverse biological functions that have been implicated in various human diseases, including LC (44-46). Global downregulation of miRNAs expression was observed in different human cancer types (47-49) after exposure to CS (50-52) or to the hormone E2 (53-56). Izzotti et al. evaluated miRNA expression in the lungs of rats exposed to CS and treated with several cancer chemopreventive agents. Administration of the dietary agents PEITC and I3C attenuated the CS-induced down-regulation of miRNA expression (57). In the case of the combined treatment with PEITC and I3C, they had profound effects on almost all CS- down-regulated miRNAs and their expression even exceeded the baseline situation (57). We have previously suggested that this effect may be related to the observed anti-estrogenic functions of PEITC and I3C (58-61). Recently, we described an association between the comprehensive miRNA repression observed in cancer and after E2 exposure, with G enrichment in the terminal loops of their precursors (62,63), which was also associated with their tendency to act as tumor suppressor miRNAs in lung and breast cancers (64). Thus, it is plausible that the effect of cruciferous dietary phytochemicals on miRNA expression may also involve the aforementioned mechanism (65,66).
Conclusions
The studies described above show that phytochemical compounds, such as those found in cruciferous vegetables, can help attenuate the molecular effects of carcinogenic substances such as CS and E2, and potentially reduce the risk of developing LC and other types of cancer. A potential application for these results may be the use of cruciferous phytochemicals as cancer preventive agents (e.g., as nutritional supplements). It is well established, however, that increasing cruciferous vegetables intake in the diet can be a simple and effective way for cancer prevention.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jxym.2019.04.01). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin 2017;67:7-30. [Crossref] [PubMed]
- Greenfield DM. A growing epidemic: cancer treatment consequences. Curr Opin Support Palliat Care 2017;11:179-80. [Crossref] [PubMed]
- Bray F, Ferlay J, Soerjomataram I, et al. Global Cancer Statistics 2018: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2018;68:394-424. [Crossref] [PubMed]
- Hecht SS. Lung carcinogenesis by tobacco smoke. Int J Cancer 2012;131:2724-32. [Crossref] [PubMed]
- Cohen AJ. Air pollution and lung cancer: what more do we need to know? Thorax 2003;58:1010-2. [Crossref] [PubMed]
- Fucic A, Gamulin M, Ferencic Z, et al. Lung Cancer and Environmental Chemical Exposure: A Review of Our Current State of Knowledge with Reference to the Role of Hormones and Hormone Receptors as an Increased Risk Factor for Developing Lung Cancer in Man. Toxicol Pathol 2010;38:849-55. [Crossref] [PubMed]
- Fajersztajn L, Veras M, Barrozo LV, et al. Air pollution: a potentially modifiable risk factor for lung cancer. Nat Rev Cancer 2013;13:674-8. [Crossref] [PubMed]
- Loomis D, Huang W, Chen G. The International Agency for Research on Cancer (IARC) evaluation of the carcinogenicity of outdoor air pollution: focus on China. Chin J Cancer 2014;33:189-96. [Crossref] [PubMed]
- Hamra GB, Guha N, Cohen A, et al. Outdoor particulate matter exposure and lung cancer: a systematic review and meta-analysis. Environ Health Perspect 2014;122:906-11. [Crossref] [PubMed]
- Eckel SP, Cockburn M, Shu YH, et al. Air Pollution Affects Lung Cancer Survival. Thorax 2016;71:891-8. [Crossref] [PubMed]
- Peng J, Xu X, Mace BE, et al. Estrogen metabolism within the lung and its modulation by tobacco smoke. Carcinogenesis 2013;34:909-15. [Crossref] [PubMed]
- Loomis D, Grosse Y, Lauby-Secretan B, et al. The carcinogenicity of outdoor air pollution. Lancet Oncol 2013;14:1262-3. [Crossref] [PubMed]
- DeMarini DM. Genotoxicity biomarkers associated with exposure to traffic and near-road atmospheres: a review. Mutagenesis 2013;28:485-505. [Crossref] [PubMed]
- Klaunig JK, Wang Z, Farombi EO. Carcinogenicity. In: Riviere JE, Monteiro-Riviere NA. editors. Reference Module in Biomedical Sciences. Elsevier, 2014.
- Strzelczyk JK, Wiczkowski A. Oxidative damage and carcinogenesis. Contemp Oncol (Pozn) 2012;16:230-3. [Crossref] [PubMed]
- Kawanishi S, Hiraku Y, Oikawa S. Mechanism of guanine-specific DNA damage by oxidative stress and its role in carcinogenesis and aging. Mutat Res 2001;488:65-76. [Crossref] [PubMed]
- Torgovnick A, Schumacher B. DNA repair mechanisms in cancer development and therapy. Front Genet 2015;6:157. [Crossref] [PubMed]
- Delaney S, Jarem DA, Volle CB, et al. Chemical and biological consequences of oxidatively damaged guanine in DNA. Free Radic Res 2012;46:420-41. [Crossref] [PubMed]
- Surh YJ. Cancer chemoprevention with dietary phytochemicals. Nat Rev Cancer 2003;3:768-80. [Crossref] [PubMed]
- Gullett NP, Ruhul Amin AR, Bayraktar S, et al. Cancer prevention with natural compounds. Semin Oncol 2010;37:258-81. [Crossref] [PubMed]
- Murillo G, Mehta RG. Cruciferous vegetables and cancer prevention. Nutr Cancer 2001;41:17-28. [Crossref] [PubMed]
- Higdon JV, Delage B, Williams DE, et al. Cruciferous vegetables and human cancer risk: epidemiologic evidence and mechanistic basis. Pharmacol Res 2007;55:224-36. [Crossref] [PubMed]
- Herr I, Büchler MW. Dietary constituents of broccoli and other cruciferous vegetables: implications for prevention and therapy of cancer. Cancer Treat Rev 2010;36:377-83. [Crossref] [PubMed]
- Bosetti C, Filomeno M, Riso P, et al. Cruciferous vegetables and cancer risk in a network of case-control studies. Ann Oncol 2012;23:2198-203. [Crossref] [PubMed]
- Zhang Z, Bergan R, Shannon J, et al. The Role of Cruciferous Vegetables and Isothiocyanates for Lung Cancer Prevention: Current Status, Challenges, and Future Research Directions. Mol Nutr Food Res 2018;62:e1700936. [Crossref] [PubMed]
- Lam TK, Gallicchio L, Lindsley K, et al. Cruciferous vegetable consumption and lung cancer risk: a systematic review. Cancer Epidemiol Biomarkers Prev 2009;18:184-95. [Crossref] [PubMed]
- Lampe JW, Peterson S. Brassica, biotransformation and cancer risk: genetic polymorphisms alter the preventive effects of cruciferous vegetables. J Nutr 2002;132:2991-4. [Crossref] [PubMed]
- Brennan P, Hsu CC, Moullan N, et al. Effect of cruciferous vegetables on lung cancer in patients stratified by genetic status: a mendelian randomisation approach. Lancet 2005;366:1558-60. [Crossref] [PubMed]
- Mori N, Shimazu T, Sasazuki S, et al. Cruciferous Vegetable Intake Is Inversely Associated with Lung Cancer Risk among Current Nonsmoking Men in the Japan Public Health Center (JPHC) Study. J Nutr 2017;147:841-9. [Crossref] [PubMed]
- Tang L, Zirpoli GR, Jayaprakash V, et al. Cruciferous vegetable intake is inversely associated with lung cancer risk among smokers: a case-control study. BMC Cancer 2010;10:162. [Crossref] [PubMed]
- Arumugam A, Abdull Razis AF. Apoptosis as a Mechanism of the Cancer Chemopreventive Activity of Glucosinolates: a Review. Asian Pac J Cancer Prev 2018;19:1439-48. [PubMed]
- Soundararajan P, Kim JS. Anti-Carcinogenic Glucosinolates in Cruciferous Vegetables and Their Antagonistic Effects on Prevention of Cancers. Molecules 2018; [Crossref] [PubMed]
- Izzotti A, Bagnasco M, Cartiglia C, et al. Modulation of multigene expression and proteome profiles by chemopreventive agents. Mutat Res 2005;591:212-23. [Crossref] [PubMed]
- Izzotti A, Balansky RM, Dagostini F, et al. Modulation of biomarkers by chemopreventive agents in smoke-exposed rats. Cancer Res 2001;61:2472-9. [PubMed]
- Hecht SS, Trushin N, Rigotty J, et al. Complete inhibition of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone-induced rat lung tumorigenesis and favorable modification of biomarkers by phenethyl isothiocyanate. Cancer Epidemiol Biomarkers Prev 1996;5:645-52. [PubMed]
- Wang H, Wang L, Cao L, et al. Inhibition of autophagy potentiates the anti-metastasis effect of phenethyl isothiocyanate through JAK2/STAT3 pathway in lung cancer cells. Mol Carcinog 2018;57:522-35. [Crossref] [PubMed]
- Izzotti A, Kanitz S, D'Agostini F, et al. Formation of adducts by bisphenol A, an endocrine disruptor, in DNA in vitro and in liver and mammary tissue of mice. Mutat Res 2009;679:28-32. [Crossref] [PubMed]
- Yager JD. Mechanisms of estrogen carcinogenesis: The role of E2/E1-quinone metabolites suggests new approaches to preventive intervention-A review. Steroids 2015;99:56-60. [Crossref] [PubMed]
- Aggarwal BB, Ichikawa H. Molecular targets and anticancer potential of indole-3-carbinol and its derivatives. Cell Cycle 2005;4:1201-15. [Crossref] [PubMed]
- Song JM, Qian X, Molla K, et al. Combinations of indole-3-carbinol and silibinin suppress inflammation-driven mouse lung tumorigenesis by modulating critical cell cycle regulators. Carcinogenesis 2015;36:666-75. [Crossref] [PubMed]
- Jiang LL, Zhou SJ, Zhang XM, et al. Sulforaphane suppresses in vitro and in vivo lung tumorigenesis through downregulation of HDAC activity. Biomed Pharmacother 2016;78:74-80. [Crossref] [PubMed]
- Wang DX, Zou YJ, Zhuang XB, et al. Sulforaphane suppresses EMT and metastasis in human lung cancer through miR-616-5p-mediated GSK3β/β-catenin signaling pathways. Acta Pharmacol Sin 2017;38:241-51. [Crossref] [PubMed]
- Dacosta C, Bao Y. The Role of MicroRNAs in the Chemopreventive Activity of Sulforaphane from Cruciferous Vegetables. Nutrients 2017; [Crossref] [PubMed]
- Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell 2009;136:215-33. [Crossref] [PubMed]
- Li Y, Kowdley KV. MicroRNAs in common human diseases. Genomics Proteomics Bioinformatics 2012;10:246-53. [Crossref] [PubMed]
- Castro D, Moreira M, Gouveia AM, et al. MicroRNAs in lung cancer. Oncotarget 2017;8:81679-85. [Crossref] [PubMed]
- Lu J, Getz G, Miska EA, et al. MicroRNA expression profiles classify human cancers. Nature 2005;435:834-8. [Crossref] [PubMed]
- Ozen M, Creighton CJ, Ozdemir M, et al. Widespread deregulation of microRNA expression in human prostate cancer. Oncogene 2008;27:1788-93. [Crossref] [PubMed]
- Dvinge H, Git A, Gräf S, et al. The shaping and functional consequences of the microRNA landscape in breast cancer. Nature 2013;497:378-82. [Crossref] [PubMed]
- Izzotti A, Calin GA, Arrigo P, et al. Downregulation of microRNA expression in the lungs of rats exposed to cigarette smoke. FASEB J 2009;23:806-12. [Crossref] [PubMed]
- Schembri F, Sridhar S, Perdomo C, et al. MicroRNAs as modulators of smoking-induced gene expression changes in human airway epithelium. Proc Natl Acad Sci U S A 2009;106:2319-24. [Crossref] [PubMed]
- Graff JW, Powers LS, Dickson AM, et al. Cigarette smoking decreases global microRNA expression in human alveolar macrophages. PLoS One 2012;7:e44066. [Crossref] [PubMed]
- Maillot G, Lacroix-Triki M, Pierredon S, et al. Widespread estrogen-dependent repression of micrornas involved in breast tumor cell growth. Cancer Res 2009;69:8332-40. [Crossref] [PubMed]
- Yu X, Zhang X, Dhakal IB, et al. Induction of cell proliferation and survival genes by estradiol-repressed microRNAs in breast cancer cells. BMC Cancer 2012;12:29. [Crossref] [PubMed]
- Cohen A, Smith Y. Estrogen regulation of microRNAs, target genes, and microRNA expression associated with vitellogenesis in the zebrafish. Zebrafish 2014;11:462-78. [Crossref] [PubMed]
- Cohen A, Burgos-Aceves MA, Smith Y. Estrogen repression of microRNA as a potential cause of cancer. Biomed Pharmacother 2016;78:234-8. [Crossref] [PubMed]
- Izzotti A, Calin GA, Steele VE, et al. Chemoprevention of cigarette smoke-induced alterations of MicroRNA expression in rat lungs. Cancer Prev Res (Phila) 2010;3:62-72. [Crossref] [PubMed]
- Cohen A, Burgos-Aceves MA, Smith Y. A potential role for estrogen in cigarette smoke-induced microRNA alterations and lung cancer. Transl Lung Cancer Res 2016;5:322-30. [Crossref] [PubMed]
- Cohen A, Burgos-Aceves MA, Smith Y. Global microRNA downregulation: all roads lead to estrogen. J Xiangya Med 2017;2:59. [Crossref]
- Meng Q, Yuan F, Goldberg ID, et al. Indole-3-carbinol is a negative regulator of estrogen receptor-alpha signaling in human tumor cells. J Nutr 2000;130:2927-31. [Crossref] [PubMed]
- Kang L, Ding L, Wang ZY. Isothiocyanates repress estrogen receptor alpha expression in breast cancer cells. Oncol Rep 2009;21:185-92. [PubMed]
- Cohen A, Burgos-Aceves MA, Kahan T, et al. Estrogen repression of microRNAs is associated with high guanine content in the terminal loop sequences of their precursors. Biomedicines 2017; [Crossref] [PubMed]
- Cohen A, Burgos-Aceves MA, Smith Y. microRNAs downregulation in cancer is associated with guanine enrichment in the terminal loop sequences of their precursors. MicroRNA 2018;7:20-7. [Crossref] [PubMed]
- Cohen A, Burgos-Aceves MA, Smith Y. Guanine content of microRNAs is associated with their tumor suppressive and oncogenic roles in lung and breast cancers. BioRxiv. 2019. Available online: https://www.biorxiv.org/content/10.1101/518472v2
- Cohen A, Burgos-Aceves MA, Smith Y. Guanine content of precursor microRNA’s terminal loop and its association with cancer. J Xiangya Med 2018;3:33. [Crossref]
- Cohen A, Burgos-Aceves MA, Smith Y. Guanine residues of precursor microRNA’s terminal loop as a potential target for cancer therapy and prevention. Precis Cancer Med 2019;2:2. [Crossref]
Cite this article as: Cohen A, Burgos-Aceves MA, Bar-Ziv N, Smith Y. Cruciferous vegetables consumption and lung cancer prevention: epidemiological studies and molecular mechanisms. J Xiangya Med 2019;4:21.