
Cancer risk in achalasia
Introduction
Achalasia is a rare esophageal motility disorder, causing a variety of symptoms including dysphagia, retrosternal pain, burning, cramping, regurgitation, and vomiting (1,2). The initial onset of symptoms is often mild to moderate in nature. Patients may not experience impairment in daily activities for months to years. The etiology of the disease is related to deterioration of the myenteric plexus in the esophageal wall, leading to loss of peristalsis and inability to relax the lower esophageal sphincter (LES). The latter causes obstruction at the cardia and, in conjunction with the loss of esophageal propulsive contraction power, gradual widening of the esophagus. Untreated, the esophagus may become progressively more dilated. As a result, the weight of retained food within the distal esophagus causes esophageal lengthening and compression against the diaphragm, leading to an s-shaped kinking at the hiatus. In the end stages of achalasia, the esophagus will have several kinks in the mediastinum seen on imaging as sigmoid widening with a curved shape. At this stage, normal transport of fluids and food is not possible, resulting in stasis and fermentation within the esophageal lumen (1,2).
As mentioned, the clinical presentation of achalasia is variable; symptoms can develop slowly or manifest as sudden dysphagia and/or thoracic pain. Degree of symptoms are best summarized using the Eckardt score (3).
The exact pathophysiology of achalasia remains unclear; results of studies showing the loss of myenteric neurons in connection with chronic ganglionitis and involvement of herpes simplex virus or human papilloma virus are controversial (2,4-7).
Achalasia is diagnosed through a combination of endoscopy, radiography and high-resolution manometry (HRM). The latter confirms the diagnosis, and the combination of the three provides staging and degree of severity of the disease. In conjunction, these three modalities allow exclusion of malignancy. HRM demonstrates major functional defects including impaired relaxation of the LES and loss of peristalsis. It also permits categorization according to the Chicago Classification (CC) (8-10). Barium esophagram delineates anatomical changes important for staging disease progression and assisting therapeutic decision making (1,2). Finally, endoscopy is essential as it permits exclusion of structural abnormalities, such as strictures or malignancy of the cardia or the esophagus, which may mimic achalasia. Secondary achalasia due to a tumor of the cardia is catastrophic for the patient, if not recognized early in the diagnostic process.
Current treatment options for achalasia have expanded in the last decade with the introduction of peroral endoscopic myotomy (POEM) (11). Medical therapy has proven to be quite ineffective and is usually abandoned. Botox injection temporizes symptoms in patients unfit for interventional or surgical therapy, but these are few.
The mainstays of achalasia treatment today include pneumatic dilatation of the LES, transesophageal myotomy using the POEM-technique, and laparoscopic myotomy combined with partial fundoplication (1,2,12,13).
The long-term trajectory of patients with achalasia varies, depending on modality and efficacy of treatment. A subset of patients develops secondary reflux after endoscopic or surgical weakening of the LES, and in those treated insufficiently, esophageal stasis persists and can lead to the worst case scenario: progression to malignancy (2).
The observation of esophageal malignancy in achalasia patients was highlighted in the literature many years ago and has continued through the present (2,14-19). The development of squamous-cell cancer in the esophagus is thought to arise from stasis of food in the dilated lumen. This leads to bacterial overgrowth and fermentation, ultimately resulting in chemical irritation of the esophageal mucosa. These patients may develop chronic retention esophagitis, dysplasia and eventually cancer (1,2,20,21). Esophageal adenocarcinoma is thought to result from the development of reflux after achalasia treatment. Subsequent chronic reflux esophagitis leads to Barrett’s esophagus, and progression to adenocarcinoma of the esophagus (21,22).
The reported incidence of malignancy in the setting of achalasia remains controversial. Our literature review demonstrated a broad range, with reports of anywhere between a 5- to 50-fold increased risk in comparison to the normal population (2,21).
Here we provide a comprehensive literature review, synthesizing the reported prevalence and incidence of malignancy in a standardized fashion, to highlight the variability between data sets.
Methods
A literature review was performed evaluating representative studies within the last 60 years on patients with achalasia, with follow-up regarding progression to malignancy, including both squamous cell carcinoma (SCC) and adenocarcinoma (AC). The review and selection of studies was based on the PRISMA project statements (23).
We searched PubMed for studies published in the English language. The following search terms were used: “achalasia and cancer” and “esophageal cancer and achalasia”. In the subsequent selection process duplicates were excluded, and series with detailed data sets were selected. The search period ranged from 60 years back through August 2018, with the earliest selected publication dated 1963.
Exclusion criteria are as follows: studies with no data regarding follow-up period or data regarding potential malignant transformation, studies including patients with previous esophageal or gastric resections such as Merendino operations, studies for which no full text article was available, studies from which no detailed data set could be obtained, and no publications such as letters, reviews, editorials or meeting proceedings.
Inclusion criteria were: documented patient data with esophageal achalasia independent of the applied therapy, followed for a certain time segment; studies with clear documentation of cancer cases occurring during follow up, permitting calculation of prevalence and incidence; studies with retrospective and prospective documentation of data; and studies with patient populations >30.
The documented outcomes were summarized in a table showing the prevalence and incidence of esophageal carcinoma, both SCC and adenocarcinoma, displayed in comparable units, as well as the time period between onset of symptoms and malignant transformation.
We encountered significant variability in our literature review. Patient follow-up was documented in both mean and median time segments. It was frequently challenging to characterize documented malignancies as prevalence vs. incidence. In some of the literature it was unclear whether patients developed cancer over the course of the study, or were initially diagnosed at the outset Malignant transformation was reported with significant variability, i.e., cancer patient per number of years, cancer patient per 1,000 patients at risk per year, or number cancer patients in a certain population. Initially we considered a separate analysis of development of esophageal SCC vs. adenocarcinoma in achalasia patients, but encountered too few publications with a clear distinction between the two entities.
In order to analyze and present the data in a cohesive fashion, we standardized the data by calculating the number of cancer cases per 100,000 patients at risk per year. A median was then calculated from the selected publications. Outliers were identified and possible causes for these results were investigated.
We analyzed duration of follow-up with cancer incidence to detect any correlation. As there has historically been tremendous variability in reported cancer incidence, the consequences for the involved patients with such conditions remain unclear. This variability resulted in one study concluding that surveillance is unnecessary, to others concluding surveillance should mandated (23-26).
Results
For the systematic review, 1,913 abstracts were screened and selected according to the PRISMA guidelines using the inclusion and exclusion criteria mentioned above. The results are demonstrated in Figure 1. A total of 26 studies were included in the quantitative evaluation. The main data are shown in Table 1, demonstrating the detailed results of the evaluation of relevant literature (14,15,24-46). Several previously published reviews and one meta-analysis from Tustumi et al. (21) were consulted to confirm the analysis (22,24-47).
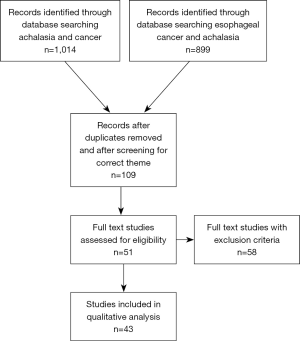
Table 1
| First author, year, country | Number of patients | Mean/median follow-up in years | Number of events of cancer | Prevalence, % | Incidence: patient-years followed/incidence per 100,000 patients (per year) | Time segments: onset symptoms (OS)-diagnosis of achalasia (DA)-cancer diagnosis (Ca) |
|---|---|---|---|---|---|---|
| Belsey, 1963, UK | 94 | 2 years (e) | 4 develop, 4 prevalent | 8.5 | 188 pt-years; 2,120 | – |
| Barrett, 1964, UK | 120 | 2 years (e) | 7 | 5.8 | 154 pt-years; 4,545 | OS-DA: 2 years |
| Wychulis, 1971, USA | 1,318 | 13 years | 7 develop, 6 prevalent | 0.1 | 17,134 pt-years; 41 | OS-DA: 19.8 years, DA-Ca: 8.6 years |
| Chuong, 1984, USA | 91 | 4 years (e) | 0 | 0 | 364 pt-years; 0 | – |
| Adeyemo, 1987, Nigeria | 33 | 7 years | 1 | 3 | 231 pt-years; 433 | OS-Ca: 4–25 years |
| Perrachia, 1991, Italy | 244 | 3.7 years | 1 | 0.41 | 907 pt-years; 110 | DA-Ca: 11.5 years |
| Aggestrup, 1992, Denmark | 146 | 23.2 years | 10 | 6.8 | 3,387 pt-years; 295 | – |
| Meijssen, 1992, Netherlands | 195 | 4.5 years | 3 | 1.5 | 874 pt-years; 343 | OS-Ca: 17 years, DA-Ca: 5.7 years |
| Arber, 1993, Israel | 162 | 10 years (e) | 0 | 0 | 1,620 pt-years; 0 | – |
| Streitz, 1995, USA | 241 | 15 years (e) | 3 develop, 6 prevalent | 3.7 | 3,615 pt-years; 83 | – |
| Sandler, 1995, Sweden | 1,062 | 9.3 years | 24 | 2.26 | 9,864 pt-years; 243 | DA-Ca: 14 years |
| DiSimone, 1996, Italy | 129 | 8.1 years | 1 | 0.7 | 1,045 pt-years; 96 | DA-Ca: 8 years |
| Horvath, 1996, Hungary | 50 | 8 years (e) | 4 | 8 | 400 pt-years; 1,000 | – |
| Brücher, 2001, Germany | 124 | 5.6 years | 4 | 3.2 | 694 pt-years; 576 | – |
| West, 2002, Netherlands | 157 | 12 years | 6 | 3.8 | 1,884 pt-years; 318 | – |
| Liu, 2004, China | 58 | 14 years | 3 | 5.2 | 812 pt-years; 369 | OS-DA: 4.8 years, DA-Ca: 17 years |
| Ruffato, 2006, Italy | 173 | 9.1 years | 4 | 2.3 | 1,571 pt-years; 255 | – |
| Csendes, 2006, Chile | 67 | 15 years (e) | 3 | 4.5 | 991 pt-years; 303 | – |
| Zendehdel, 2007, Sweden | 2,896 | 9.9 years | 22 | 0.76 | 25,766 pt-years; 85 | OS-DA: 2.6 years |
| Zaninotto, 2008, Italy | 226 | 18.3 years | 4 | 1.77 | 4,136 pt-years; 96 | OS-DA: 5–10 years, DA-Ca: 10.5 years |
| Eckardt, 2008, Germany (prospective group) | 177 | 9.3 years | 0 | 0 | 1,646 pt-years; 0 | OS-DA: 1.5 years |
| Eckardt, 2008, Germany (prevalent group) | 76 | 9.2 years | 2 | 2.6 | 699 pt-years; 286 | DA-Ca: 17.2 years, OS-Ca: 24.5 years |
| Leeuwenburgh, 2010, Netherlands | 448 | 11 years | 15 | 3.34 | 4,483 pt-years; 334 | OS-DA: 13 years, DA-Ca: 11 years |
| Gossage, 2014, Australia | 171 | 5 years | 2 | 1.2 | 855 pt-years; 233 | – |
| Ota, 2016, Japan | 32 | 14.3 years | 6 | 18.7 | 458 pt-years; 1,311 | – |
| Ponds, 2018, Belgium | 230 | 9 years (e) | 3 | 0.87 | –; 63 | – |
(e), median follow-up is estimated from the paper.
Table 1 shows the data of a total of 8,720 patients with Achalasia, documented in 26 studies between 1963 and 2018. The studies documented a total follow-up time of 251.4 years, with median follow up time of 9.2 years (range, 2–23.2 years). The prevalence of cancer in these 8,720 patients over the follow-up time was a median of 2.45% (range, 0–18.7%).
The data on incidence was based on 83,778 patient-years at risk. We gathered both mean and medians from our literature review and standardized into patient-years in order to compare results between studies. This may have influenced our data. The median incidence reported in the studies was 270 cancer cases per 100,000 patients at risk per year (range, 0–4,545), as shown in Table 1. Four studies showed an extremely high incidence (n=1,000, 1,311, 2,120 and 4,545).
In addition, studies with extended follow-up were evaluated separately to assess whether higher cancer risk was documented with prolonged study duration in this patient population. Thus, studies with follow-up greater than 9 years were investigated separately. The median incidence was 249 cancer cases per 100,000 patients at risk per year (range, 0–1,311).
Studies with follow-up of less than 9 years were separately investigated and showed a median crude incidence rate of 398 cancer cases per 100,000 patients at risk per year.
In studies with a low incidence (<100 cancer cases per 100,000 patients at risk per year), median follow-up time was 9.9 years (range, 4–18.3 years). This is similar to the follow-up time of all studies reviewed.
In terms of clinical significance, it is important to assess the duration of time between onset of symptoms and the development of cancer. From the data available in the different studies, one can determine the time interval between “onset of symptoms” and cancer diagnosis, which ranged from 17 to 28 years with a median of 21.8 years. The time interval between the diagnosis of Achalasia and the diagnosis of cancer had a median of 10.9 years (range, 5.7–17.2 years).
When comparing achalasia patients to the normal population, we noted variability between different countries (Table 2). The incidence of esophageal carcinoma in the United States in 2009 was 16,400 new cases per 315,000,000. This equals a crude rate of approximately 53 cancer cases per 100,000 persons at risk per year. In Germany, about 7,000 new cases per year in 82,200,000 individuals can be expected, resulting in a crude rate of about 8.5 cancer cases per 100,000 persons per year. It is critical to note the differences in data reporting in different countries. Table 2 demonstrates the annual new cases of esophageal cancer in different countries (2,25,48-51). Table 1 shows the country of origin of publications, permitting comparison between cancer incidence (both squamous cell cancer and adenocarcinoma of the esophagus) in achalasia patients. In some countries, consistency of incidence rate is noted, while others demonstrated significant variability within the same country (Table 2).
Table 2
| Country | New cases per year | Population | New cases per 100,000 persons in the population | Crude incidence rate in publications from these countries in achalasia patients |
|---|---|---|---|---|
| China | 259,235 | 1,371,000,000 | 18.9 | Liu: 369 |
| Germany | 7,000 | 82,200,000 | 8.5 | Brücher: 576; Eckardt: 0; Eckardt: 286 |
| Japan | 17,500 | 127,000,000 | 13.7 | Ota: 1,311 |
| Sweden | 309 | 9,800,000 | 3.15 | Sandler: 243; Zendehdel: 85 |
| Netherlands | 1,100 | 17,000,000 | 6.5 | Meijssen: 343; West: 318; Leeuwenburgh: 334 |
| USA | 17,000 | 310,000,000 | 5.4 | Wychulis: 41; Chuong: 0; Streitz: 83 |
In summary, the incidence of cancer development in Achalasia patients ranges from 0 to 4,545 cancer cases per 100,000 patients at risk per year, with a median of 270 cancer cases per 100,000 persons at risk per year. The difference between short and long-term follow-up was minimal. However, the critical time segment to track is duration from initial onset of symptoms, as we consistently noted malignant transformation 15–20 years after development of symptomatology. Thus, patients with achalasia have increased cancer risk, secondary to multiple factors. Namely, long-term stasis in the esophageal lumen leading to esophagitis and squamous cell cancer, and in patients’ post-achalasia treatment, from weakening of the LES resulting in chronic reflux with subsequent Barrett’s esophagus and adenocarcinoma.
Discussion
The relationship between achalasia and cancer was documented almost 150 years ago, with recent publications focusing on the issue (12,14-16,19). The current overview shows that esophageal cancer incidence is increased in most series on Achalasia patients compared to the normal population (24-46). However, there are substantial differences between countries, resulting in the broad range seen in the literature. The aim of this analysis is to standardize the data for comparison in order to draw conclusions on management moving forward. Since details of data were not consistently provided to allow an age adjusted analysis, crude rates of incidences were calculated and presented.
Three important issues must be discussed: (I) Do the results clearly demonstrate a higher cancer incidence? (II) Is it warranted to suggest a surveillance program for achalasia patients? and (III) What can be done to prevent cancer development for the individual patient?
An older study from the US on over 1,300 patients showed a limited 7-fold increase in cancer risk in Achalasia patients compared to the normal population (27). A study from Israel showed no cancer development at all, following a population of patients for 10 years (24). On the other hand, Brücher et al. in Germany observed a high 140-fold increase in the risk of cancer development in 124 achalasia patients. Eckardt et al. followed 177 patients in Germany and found no increased risk (25,37). Similar variability was published by Sandler et al. in 1995 and subsequently by Zendehdel et al. in 2011, demonstrating a higher rate in the earlier publication, and only a 10-fold increase in the 2011 paper (34,43).
It is speculated that the large differences in cancer risk may be a result of the difference in follow-up time between studies. The high outliers in cancer development are usually older observational studies; one can assume that malignancies were prevalent and not diagnosed prior to the study (14,15). In contrast, Eckardt showed that in his prospective follow-up group no cancer development was observed for 9.3 years. These patients were followed from time of initial achalasia diagnosis. His second patient group consisted of individuals diagnosed with achalasia years prior, treated at outside institutions, and referred for recrudescence of symptoms. In this group, two cancers developed 14 and 20 years after initial diagnosis (25). This argument is supported by other articles (20,36,52-54). Eckardt concluded that life expectancy was not reduced in Achalasia patients compared to the normal German population (25,53).
A critical study from the Netherlands evaluated 448 patients with achalasia with a mean follow-up time of 9.6 years (26). The crude incidence rate was 334 cases per 100,000 patients per year, indicating an age-adjusted 28-fold increase compared to the normal Dutch population. The mean age at the time of diagnosis was 71 years (range, 36–90 years), supporting the argument for increased risk of malignant transformation in achalasia patients 10–15 years after the onset of the disease. Again, this highlights that duration after start of symptoms of >15 years is a critical time window, rather than time from actual diagnosis to cancer development.
In the recent systematic review and meta-analysis by Tustumi et al. the mean age of Achalasia patients diagnosed with esophageal cancer was 56.9 years for SCC and 68.3 years for AC (21). The mean period between onset of symptoms and the diagnosis of cancer was 22.2 years, supporting our argument that malignant transformation in achalasia patients occurs after >15 years of symptoms. In this meta-analysis the mean number of cancer cases per 100,000 patient-years at risk having Achalasia was 21.23. This is substantial and suggests the necessity for surveillance.
This leads to the second question: is it warranted to recommend a surveillance program for achalasia patients?
As many studies with prospective follow-up have tracked increased cancer occurrence, one would expect to see a respective increase in the opportunity for curative intervention. However, even in prospective studies, the results of cancer management are limited. Unfortunately, the tumor has often reached an advanced stage at the time of diagnosis in achalasia patients who develop cancer (25,30,32,33,37). This is often a result of the altered anatomy of the esophagus. A dilated esophagus allows the tumor to grow to a substantial size prior to development of warning symptoms such as dysphagia.
Another compounding issue is the inaccuracy of gross endoscopic exploration. Endoscopic judgement and the accuracy of focused biopsies may be limited by retained food adherent to the mucosa, thickening of the mucosa from chronic irritation/inflammation, and loss of soft tissue flexibility.
In conjunction with the limitations of symptom interpretation, the success of surveillance programs is hindered by anatomical challenges and technical expertise in locating concerning lesions. One could argue against routine surveillance for these reasons. In addition, costs for surveillance programs can be substantial and success is limited.
Based on the data presented, a surveillance program for achalasia patients is not clearly justified. However, the authors are in favor of a selective approach to surveillance.
High-risk patients include those with long-standing (>5 years) retention esophagitis, mega-esophagus, and long-term history of the disease, with >10 years since the onset of symptoms (55-62). These patients could be candidates for more frequent endoscopic follow up.
What can be done to prevent cancer development for the individual patient?
As mentioned previously, increased incidence of cancer development in achalasia patients is thought to be secondary to distal obstruction causing fluid stasis and fermentation, resulting in chronic irritation (1,2,21). Retention esophagitis leads to histologic alterations and squamous cell hyperplasia. Immunohistochemical staining of tissue from patients with retention esophagitis showed higher rates of p53 expression (63,64). Gong et al. recommended focused surveillance in patients with retention esophagitis (65,66).
Treatment failure after initial therapy for Achalasia is another criterion for selection. A Dutch study previously showed that the success rate of pneumatic dilatation did not influence the incidence of cancer development (26), suggesting that development of malignancy may not correlate with success of reducing the obstruction in the cardia.
There is more to this thinking. Though successful treatment of the mechanical barrier created by the non-relaxing LES reduces symptoms, certain patients develop secondary reflux of gastric contents into the esophagus. Reflux esophagitis and subsequent Barrett’s esophagus open a door to another cancer, adenocarcinoma. Recent studies highlight the development of adenocarcinoma in Achalasia patients after treatment (22,43,47). In one Dutch study 3 out of 15 cancers were adenocarcinoma (26,47). A similar phenomenon is reported in patients after esophagectomy and gastric pull-up reconstruction, in whom the proximal esophageal stump is exposed to acid above the anastomosis for years after the operation. This can lead to reflux esophagitis and subsequent development of adenocarcinoma (67). Thus, successful achalasia treatment may not obviate risk of cancer development. In patients’ post-achalasia treatment, a symptom score should be administered such as the Eckardt or GERD-HQRL to identify those warranting surveillance.
The most recent ISDE guidelines for Achalasia discuss the risk of cancer (2): “achalasia patients carry a moderately increased risk of development of squamous esophageal cancer 10 years or more from the primary treatment of achalasia”. Earlier studies have shown a high 140-fold rate of cancer development. In more recent studies, cancer risk was reported in the 10–50 fold range compared to the normal population. Again, bear in mind that the incidence of cancer in the “normal” population differs substantially between countries (2,25,26,46,48-51).
Recent studies recommended that “achalasia patients should be informed that a moderately increased risk of esophageal cancer is present” (21,26,32,42,43). Interestingly the consensus group of authors state that they do not make recommendations about routine endoscopy surveillance nor do they suggest interval EGDs after treatment (2).
Conclusions
In conclusion, there is reasonable evidence in the literature over the past 20 years to suggest that long-standing Achalasia patients, particularly males, and those with long-term obstruction, stasis, and retention esophagitis are at increased risk for cancer development.
Thus, surveillance endoscopy can be recommended to a selected group of patients. Furthermore, patients with documented persistent reflux esophagitis after successful treatment of achalasia should be followed to keep their GERD symptoms controlled, and for periodic evaluation for development of Barrett’s esophagus.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (David W. Rattner, Ozanan Meireles) for the series “Update on the Diagnosis and Treatment of Achalasia” published in Journal of Xiangya Medicine. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jxym.2018.12.05). The series “Update on the Diagnosis and Treatment of Achalasia” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Boeckxstaens GE, Zaninotto G, Richter JE. Achalasia. Lancet 2014;383:83-93. [Crossref] [PubMed]
- Zaninotto G, Bennett C, Boeckxstaens G, et al. The 2018 ISDE achalasia guidelines. Dis Esophagus 2018;31. [PubMed]
- Eckardt VF. Clinical presentations and complications of achalasia. Gastrointest Endosc Clin N Am 2001;11:281-92. [Crossref] [PubMed]
- Castagliuolo I, Brun P, Costantini M, et al. Esophageal achalasia: is the herpes simplex virus really innocent? J Gastrointest Surg 2004;8:24-30. [Crossref] [PubMed]
- Facco M, Brun P, Baesso I, et al. T cells in the myenteric plexus of achalasia patients show a skewed TCR repertoire and react to HSV-1 antigens. Am J Gastroenterol 2008;103:1598-609. [Crossref] [PubMed]
- Boeckxstaens GE. Achalasia: virus-induced euthanasia of neurons? Am J Gastroenterol 2008;103:1610-12. [Crossref] [PubMed]
- Birgisson S, Galinski MS, Goldblum JR, et al. Achalasia is not associated with measles or known herpes and human papilloma viruses. Dig Dis Sci 1997;42:300-6. [Crossref] [PubMed]
- Pandolfino JE, Ghosh SK, Rice J, et al. Classifying esophageal motility by pressure topography characteristics: a study of 400 patients and 75 controls. Am J Gastroenterol 2008;103:27-37. [Crossref] [PubMed]
- Pandolfino JE, Kwiatek MA, Nealis T, et al. Achalasia: a new clinically relevant classification by high-resolution manometry. Gastroenterology 2008;135:1526-33. [Crossref] [PubMed]
- Kahrilas PJ, Bredenoord AJ, Fox M, et al. The Chicago Classification of esophageal motility disorders, v3.0. Neurogastroenterol Motil 2015;27:160-74. [Crossref] [PubMed]
- Inoue H, Minami H, Kobayashi Y, et al. Peroral endoscopic myotomy (POEM) for esophageal achalasia. Endoscopy 2010;42:265-71. [Crossref] [PubMed]
- Vaezi MF, Pandolfino JE, Vela MF. ACG clinical guideline: diagnosis and management of achalasia. Am J Gastroenterol 2013;108:1238-49; quiz 1250. [Crossref] [PubMed]
- Stefanidis D, Richardson W, Farrell TM, et al. SAGES guidelines for the surgical treatment of esophageal achalasia. Surg Endosc 2012;26:296-311. [Crossref] [PubMed]
- Belsey R. Functional disease of the esophagus. Postgrad Med J 1963;39:290-8. [Crossref] [PubMed]
- Barrett NR. Achalasia of the Cardia: Reflections upon a Clincal Study of over 100 cases. Br Med J 1964;1:1135-40. [Crossref] [PubMed]
- Belsey R. Functional disease of the esophagus. J Thorac Cardiovasc Surg 1966;52:164-88. [PubMed]
- Lortat-Jacob JL, Richard CA, Fekete F, et al. Cardiospasm and esophageal carcinoma: report of 24 cases. Surgery 1969;66:969-75. [PubMed]
- Pierce WS, MacVaugh H 3rd, Johnson J. Carcinoma of the esophagus arising in patients with achalasia of the cardia. J Thorac Cardiovasc Surg 1970;59:335-9. [PubMed]
- Hankins JR, McLaughlin JS. The association of carcinoma of the esophagus with achalasia. J Thorac Cardiovasc Surg 1975;69:355-60. [PubMed]
- Lopes AB, Fagundes RB. Esophageal squamous cell carcinoma - precursor lesions and early diagnosis. World J Gastrointest Endosc 2012;4:9-16. [Crossref] [PubMed]
- Tustumi F, Bernardo WM, da Rocha JRM, et al. Esophageal achalasia: a risk factor for carcinoma. A systematic review and meta-analysis. Dis Esophagus 2017;30:1-8. [Crossref] [PubMed]
- Leeuwenburgh I, Haringsma J, Van Dekken H, et al. Long-term risk of oesophagitis, Barrett’s oesophagus and esophageal cancer in achalasia patients. Scand J Gastroenterol Suppl 2006;7-10. [Crossref] [PubMed]
- Moher D, Liberati A, Tetzlaff J, et al. Preferred Reporting items for systematic Reviews and Metaanalyses: The PRISMA statement. PLoS Med 2009;6:e1000097. [Crossref] [PubMed]
- Arber N, Grossman A, Lurie B, et al. Epidemiology of achalasia in central Israel. Rarity of esophageal cancer. Dig Dis Sci 1993;38:1920-5. [Crossref] [PubMed]
- Eckardt VF, Hoischen T, Bernhard G. Life expectancy, complications, and causes of death in patients with achalasia: results of a 33-year follow-up investigation. Eur J Gastroenterol Hepatol 2008;20:956-60. [Crossref] [PubMed]
- Leeuwenburgh I, Scholten P, Alderliesten J, et al. Long-term esophageal cancer risk in patients with primary achalasia: a prospective study. Am J Gastroenterol 2010;105:2144-9. [Crossref] [PubMed]
- Wychulis AR, Woolam GL, Andersen HA, et al. Achalasia and carcinoma of the esophagus. JAMA 1971;215:1638-41. [Crossref] [PubMed]
- Chuong JJH, DuBovic S, McCallum RW. Achalasia as a risk factor for esophageal carcinoma: a reappraisal. Dig Dis Sci 1984;29:1105-8. [Crossref] [PubMed]
- Adeyemo AO, Lawal O, Ojelade A. Achalasia of the esophagus: reflections upon a clinical study of 33 cases. J Natl Med Assoc 1987;79:65-71. [PubMed]
- Peracchia A, Segalin A, Bardini R, et al. Esophageal carcinoma and achalasia:prevalence, incidence, and results of treatment. Hepatogastroenterology 1991;38:514-6. [PubMed]
- Aggestrup S, Holm JC, Sorensen HR. Does achalasia predispose to cancer of the esophagus? Chest 1992;102:1013-6. [Crossref] [PubMed]
- Meijssen MA, Tilanus HW, van Blankenstein M, et al. Achalasia complicated by oesophageal squamous cell carcinoma: a prospective study in 195 patients. Gut 1992;33:155-8. [Crossref] [PubMed]
- Streitz JM Jr, Ellis FH Jr, Gibb SP, et al. Achalasia and squamous cell carcinoma of the esophagus: analysis of 241 patients. Ann Thorac Surg 1995;59:1604-9. [Crossref] [PubMed]
- Sandler RS, Nyrén O, Ekbom A, et al. The Risk of Esophageal Cancer in Patients With Achalasia - A Population-Based Study. JAMA 1995;274:1359-62. [Crossref] [PubMed]
- Di Simone MP, Felice V, D'Errico A, et al. Onset timing of delayed complications and criteria of follow-up after operation for esophageal achalasia. Ann Thorac Surg 1996;61:1106-10; discussion 1110-1. [Crossref] [PubMed]
- Horvath OP, Karacsonyi G, Dobronte Z, et al. Development of esophageal cancer in patients following cardiomyotomy. Langenbecks Arch Chir 1986;368:163-72. [PubMed]
- Brücher BL, Stein HJ, Bartels H, et al. Achalasia and esophageal cancer: incidence, prevalence, and prognosis. World J Surg 2001;25:745-9. [Crossref] [PubMed]
- West RL, Hirsch DP, Bartelsman JF, et al. Long term results of pneumatic dilation in achalasia followed for more than 5 years. Am J Gastroenterol 2002;97:1346-51. [Crossref] [PubMed]
- Liu JF, Zhang J, Tian ZQ, et al. Long-term outcome of esophageal myotomy for achalasia. World J Gastroenterol 2004;10:287-91. [Crossref] [PubMed]
- Ruffato A, Mattioli S, Lugaresi ML, et al. Long-term results after Heller-Dor operation for oesophageal achalasia. Eur J Cardiothorac Surg 2006;29:914-9. [Crossref] [PubMed]
- Csendes A, Braghetto I, Burdiles P, et al. Very Late Results of Esophagomyotomy for Patients With Achalasia: Clinical, Endoscopic, Histologic, Manometric, and Acid Reflux Studies in 67 Patients for a Mean Follow-up of 190 Months. Ann Surg 2006;243:196-203. [Crossref] [PubMed]
- Zaninotto G, Rizzetto C, Zambon P, et al. Long-term outcome and risk of oesophageal cancer after surgery for achalasia. Br J Surg 2008;95:1488-94. [Crossref] [PubMed]
- Zendehdel K, Nyren O, Edberg A, et al. Risk of esophageal adenocarcinoma in achalasia patients, a retrospective cohort study in Sweden. Am J Gastroenterol 2011;106:57-61. [Crossref] [PubMed]
- Gossage JA, Devitt PG, Watson DI, et al. Surveillance endoscopy at five or more years after cardiomyotomy for achalasia. Ann Surg 2014;259:464-8. [Crossref] [PubMed]
- Ota M, Narumiya K, Kudo K, et al. Incidence of Esophageal Carcinomas After Surgery for Achalasia: Usefulness of Long-Term and Periodic Follow-Up. Am J Case Rep 2016;17:845-9. [Crossref] [PubMed]
- Ponds FA, Moonen A, Smout AJPM, et al. Screening for dysplasia with Lugol chromoendoscopy in longstanding idiopathic achalasia. Am J Gastroenterol 2018;113:855-62. [Crossref] [PubMed]
- Leeuwenburgh I, Scholten P, Calje TJ, et al. Barrett’s esophagus and esophageal adenocarcinoma are common after treatment for achalasia. Dig Dis Sci 2013;58:244-52. [Crossref] [PubMed]
- Lagergren J, Mattsson F. No further increase in the incidence of esophageal adenocarcinoma in Sweden. Int J Cancer 2011;129:513-6. [Crossref] [PubMed]
- Lin Y, Totsuka Y, He Y, et al. Epidemiology of esophageal cancer in Japan and China. J Epidemiol 2013;23:233-42. [Crossref] [PubMed]
- van Nistelrooij AM, van Steenbergen LN, Spaander MC, et al. Treatment and outcome of young patients with esophageal cancer in the Netherlands. J Surg Oncol 2014;109:561-566. [Crossref] [PubMed]
- Harvey PR, Thomas T, Chandan JS, et al. Incidence, morbidity and mortality of patients with achalasia in England: findings from a study of nationwide hospital and primary care data. Gut 2018; [Epub ahead of print]. [Crossref] [PubMed]
- Dunaway PM, Wong RK. Risk and surveillance intervals for squamous sell carcinoma in achalasia. Gastrointest Endosc Clin N Am 2001;11:425-34. [Crossref] [PubMed]
- Eckardt AJ, Eckardt VF. Cancer Surveillance in Achalasia: Better Late Than Never? Am J Gastroenterol 2010;105:2150-2. [Crossref] [PubMed]
- Ríos-Galvez S, Meixueiro-Daza A, Remes-Troche JM. Achalasia: a risk factor that must not be forgotten for esophageal squamous cell carcinoma. BMJ Case Reports 2015;2015.
- O’Neill OM, Johnston BT, Coleman HG. Achalasia: A review of clinical diagnosis, epidemiology, treatment and outcomes. World J Gastroenterol 2013;19:5806-12. [Crossref] [PubMed]
- Markar SR, Mackenzie H, Askari A, et al. Population-based cohort study of surgical myotomy and pneumatic dilatation as primary interventions for oesophageal achalasia. Br J Surg 2018;105:1028-35. [Crossref] [PubMed]
- Camara-Lopes LH. Carcinoma of the esophagus as a complication of megaesophagus. An analysis of seven cases. Am J Dig Dis 1961;6:742-56. [Crossref] [PubMed]
- Brandalise NA, Leonardi LS, Della Torre CA, et al. Esophageal carcinoma in chagasic mega-esophagus after Merendino’s operation. Rev Paul Med 1974;83:169-72. [PubMed]
- Carter R, Brewer LA 3rd. Achalasia and esophageal carcinoma. Studies in early diagnosis for improved surgical management. Am J Surg 1975;130:114-20. [Crossref] [PubMed]
- Norton GA, Postlethwait RW, Thompson WM. Esophageal carcinoma: a survey of populations at risk. South Med J 1980;73:25-7. [Crossref] [PubMed]
- Bardini R, Segalin A, Bonavina L, et al. Cancer in megaesophagus:our experience. Ann Ital Chir 1990;61:249-52. [PubMed]
- Henry MA, Lerco MM, Oliveira WK. Esophageal cancer in patient with chagasic megaesophagus. Arq Gastroenterol 2007;44:151-5. [Crossref] [PubMed]
- Leeuwenburgh I, Gerrits MM, Capello A, et al. Expression of p53 as predictor for the development of esophageal cancer in achalasia patients. Dis Esophagus 2010;23:506-11. [Crossref] [PubMed]
- Kim H, Park H, Choi H, et al. Retention Esophagitis as a Significant Clinical Predictor of Progression to Esophageal Cancer in Achalasia. Clin Endosc 2018;51:161-6. [Crossref] [PubMed]
- Gong EJ, Kim DH. Retention Esophagitis in Patients with Achalasia Requires Cancer Surveillance. Clin Endosc 2018;51:111-2. [Crossref] [PubMed]
- Ravi K, Geno DM, Katzka DA. Esophageal cancer screening in achalasia: is there a consensus? Dis Esophagus 2015;28:299-304. [Crossref] [PubMed]
- da Rocha JR, Ribeiro U Jr, Sallum RA, et al. Barrett’s esophagus and carcinoma in the esophageal stump after esophagectomy with gastric pull-up in achalasia patients: a study based on 10 years follow-up. Ann Surg Oncol 2008;15:2903-9. [Crossref] [PubMed]
Cite this article as: Lee A, Fuchs KH, Horgan S. Cancer risk in achalasia. J Xiangya Med 2019;4:4.


