Management of bone metastases
Introduction
Bone is a common site of metastasis for many solid malignancies, in particular, those arising from the lung, breast, prostate, thyroid, and kidney. These account for approximately 80% of all skeletal metastases. Other malignant primary tumors which metastasize to bone include but are not limited to melanomas, gastrointestinal as well as gynecologic cancers. Bone may also be a secondary site of disease involvement in hematologic malignancies such as lymphoma and multiple myeloma. Although the exact incidence of bone metastases is not known, it is thought that more than half of all cancer patients who eventually succumb to their disease have some degree of bone involvement. The preferential localization and growth of tumor cells in bone has been the subject of considerable research. The prevailing hypothesis, the so-called “seed and soil hypothesis”, describes complex interactions between adhesive molecules either produced or expressed by tumor cells and factors in the bone microenvironment.
Based on their radiologic appearance, bone metastases are broadly categorised as either osteolytic, where resorptive processes predominate, or osteoblastic, where there is deposition of new bone. Certain tumor types are characterized by either osteoblastic or osteolytic patterns. For instance, osteoblastic metastases predominate in prostate and breast cancer. On the other hand, metastases from multiple myeloma, as well as those arising from lung, kidney, and thyroid cancers are primarily lytic. In addition, mixed lytic and sclerotic patterns occur in approximately 25% of breast cancers and 15% of prostate and lung cancers. In general, osteolytic metastases are more likely to cause skeletal complications such as pathologic fractures and hence, tend to have symptomatic manifestations earlier than osteoblastic metastases.
Although bone metastases could, in theory, occur at any skeletal site, the most common locations are the vertebral column (mobile spine and sacrum), pelvis, proximal femora, and skull. Within the spine, the lumbar spine is most commonly affected followed by the thoracic and cervical spine. Lesions in the distal appendicular skeleton are exceedingly rare and when they do arise here, they are most commonly associated with lung and renal cell cancers.
Clinical presentation
Many bone metastases cause no symptoms and are diagnosed incidentally during staging investigations. In symptomatic patients, bone metastases represent a significant source of morbidity. Depending on their location, they cause a wide range of symptoms and can adversely affect function, quality of life, and survival. Direct effects of skeletal involvement by malignancy include severe pain, pathologic fractures, and neurologic symptoms from nerve root, spinal cord, or cauda equina compression. These can range from radicular pain and sensory deficits to ataxia, motor weakness progressing to paralysis, as well as bowel and bladder dysfunction.
In addition to these local complications, osteolytic metastases can result in life-threatening hypercalcemia. Clinical symptoms of hypercalcemia vary depending upon the degree of hypercalcemia as well as the rate of elevation of serum calcium levels. In addition, at any particular level of hypercalcemia, there are interindividual variations in the manifestation of symptoms, with elderly patients being particularly vulnerable to its effects. Patients with mildly elevated serum calcium concentrations may be asymptomatic particularly if the elevation in levels is chronic. Those with moderately elevated serum calcium levels may exhibit polydipsia, polyuria, anorexia, nausea, and constipation. With higher elevations in serum calcium concentration, symptoms progress to include weakness and changes in sensorium, culminating in coma and death, if levels are uncontrolled.
Diagnostic approach
Many bone metastases are asymptomatic and are diagnosed incidentally during the initial staging evaluation of the primary tumor. When a patient with a known malignancy, with or without a prior diagnosis of bone metastases, presents with progressive bone pain, some form of focused imaging is warranted. The choice of imaging should be guided by the underlying tumor type (where osteolytic or osteoblastic metastases may predominate) and the clinical presentation. For instance, for pain in the extremities, plain radiographs of the affected region is usually performed as the initial evaluation. However, a normal X-ray does not exclude an underlying bone metastasis as significant bone destruction needs to be present before a lesion is appreciable on plain radiographs. Hence, further evaluation with cross-sectional imaging, i.e., CT or MRI of the involved extremity should be considered depending on the degree of clinical suspicion.
For cancer patients presenting with back pain, contrast-enhanced MRI spine is indicated to evaluate bone metastases, extent of epidural disease and presence of nerve root or spinal cord compression. In some instances, it is useful in differentiating between bone metastasis and osteomyelitis/spondylodiscitis. CT scans of the spine, on the other hand, are superior at evaluating structural integrity of bone and hence, often performed by the operating surgeon when spine stabilization or decompression is being considered. While bone scans and PET/CT scans are often used in the initial staging evaluation in a patient with recently diagnosed malignancy, they are less commonly used to investigate bone pain in patients with known malignancy.
The need for biopsy depends on whether a radiologic diagnosis is sufficient or if histopathologic diagnosis is required to confirm metastasis. For patients without a prior diagnosis of metastatic disease in whom the index cancer has been in remission, pathologic diagnosis is almost always necessary to confirm recurrence. For patients with a background of stage IV cancer, clinical diagnosis with one or more imaging modalities is usually sufficient to make the diagnosis of bone metastasis.
Therapeutic options
Management of patients with metastatic bone disease is multimodal and requires an integrated multidisciplinary approach involving radiation oncologists, medical oncologists, palliative care physicians, orthopedic and neurosurgeons, radiologists, nurses, social workers, and physiotherapists to name a few. The goals of management include symptom control, preservation and/or restoration of function, risk reduction of skeletal-related events (SREs), e.g., fractures, hypercalcemia etc., skeletal stabilization as well as local tumor control.
Factors influencing choice of treatment include severity of symptoms, clinical disease status (widespread metastatic disease versus oligometastatic disease), estimated life expectancy, patient performance status as well as patient preferences. For patients with long bone or vertebral metastases, the decision-making process about whether to pursue surgical versus nonsurgical management can be complex. Various decision framework models and scoring systems are utilized in clinical practice to aid this process and these will be discussed in greater detail in the ensuing sections
Pharmacologic management
There are a variety of pharmacologic approaches to treat pain arising from bone metastases. These include analgesics, bone-modifying agents, as well as radiopharmaceuticals and each of these will be discussed in turn in the following section
Bone-modifying agents
The use of bone-modifying agents, also known as osteoclast inhibitors has been shown to significantly reduce SREs in patients with osseous metastases arising from a variety of solid tumors. SREs are clinically measurable outcomes or complications arising from bone metastases and include pathologic fractures, spinal cord compression, hypercalcemia of malignancy, as well as the necessity for radiotherapy or surgery to bone due to pain or impending or actual fractures. There are two broad classes of bone-modifying agents utilized for osteoclast inhibition in patients with skeletal metastases: bisphosphonates and denosumab. The selection of one agent over another is generally dictated by tumor type as well as physician and patient preference which may, in turn, be influenced by factors such as route and frequency of drug administration as well as cost of treatment.
Denosumab is a monoclonal antibody against RANKL (receptor activator of nuclear factor kappa B ligand), a key component in the pathway for osteoclast formation and activation. By targeting this receptor, inhibition of bone resorption is achieved. Denosumab has been shown to effectively reduce the risk of SREs in patients with bone metastases from a wide variety of solid tumors. In a meta-analysis comparing denosumab with zoledronic acid, denosumab was found to be superior in reducing the risk of first SRE and in delaying the time to first SRE although progression-free and overall survival rates were similar in both arms (1). Denosumab has also been shown to have a modest but statistically significant benefit over zoledronic acid, from the standpoint of pain and health-related QOL (2,3). It is given as monthly subcutaneous injections and this route of administration may be more convenient and preferred in some patients. However, it is important to note that denosumab is more expensive than bisphosphonates and this may be a consideration in patients with financial constraints.
If a bisphosphonate is chosen, zoledronic acid is generally preferred as it has proven efficacy in multiple tumor types, is considered to be most potent of all the bisphosphonates to date, requires a shorter infusion time compared with older bisphosphonates such as pamidronate and there is literature in certain cancer types such as breast and prostate cancer to support a dosing interval of 12 weeks instead of 4 weeks (4). In bone metastases from lung cancer and from solid tumors other than breast and prostate cancer, where there is limited comparative data favoring a less frequent dosing schedule, and in patients with extensive and/or highly symptomatic bone metastases, four-weekly administration may still be preferred.
Both bisphosphonates and denosumab are generally well-tolerated but they are associated with potentially serious side-effects such as jaw osteonecrosis and hypocalcaemia. Other adverse effects specific to bisphosphonates include a self-limiting flu-like syndrome, renal impairment, and an increased risk of atrial fibrillation and stroke while patients on denosumab have an increased risk of infection.
For this reason, while the use of bone-modifying agents is recommended in most patients with bone metastases from solid tumors, risk-benefit ratios for the use of these drugs generally do not favor patients with minimal bone tumor burden in whom imminent skeletal complications are considered unlikely as well as patients with limited life expectancies.
For patients in whom osteoclast inhibition is indicated, pre-treatment dental evaluation to optimize dental health is key. In addition, patients should be counselled regarding adequate vitamin D and calcium intake during therapy and pre-existing deficiencies should be corrected. Renal function should also be evaluated and assessed periodically throughout therapy. In the absence of excessive toxicity, treatment is generally continued indefinitely even in patients who experience SREs while on osteoclast-inhibiting therapy.
Analgesia
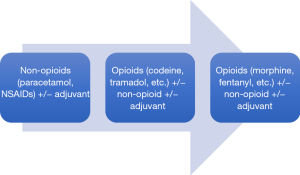
A range of pharmacologic agents are commercially available to treat cancer-related bone pain. They should be viewed as an adjunct to tumor-directed treatment rather than as a replacement. Figure 1 illustrates the WHO-endorsed stepwise approach to cancer pain management (http://www.who.int/cancer/palliative/painladder/en/).
Paracetamol and non-steroidal anti-inflammatory drugs (NSAIDs) are non-opioid analgesics that are frequently used as the initial treatment of mild to moderate cancer-related pain. For moderate to severe cancer-related pain, opioids form the cornerstone of management as they are effective for most types of cancer pain. Oral morphine is the most commonly used initial agent and has the longest history of use in the management of chronic cancer pain. However, randomized trials and systematic reviews have not demonstrated superiority of morphine over other mu agonists such as oxycodone, hydromorphone, or fentanyl and choice of opioid is generally dictated by a number of factors such as clinician preference/familiarity, prior patient experiences, cost, dosing implications as well as presence of co-morbidities such as renal insufficiency (5-8). For instance, transdermal fentanyl is generally preferred over an orally administered opioid in patients with significant dysphagia or poor anticipated drug absorption from the gastrointestinal tract. Its use is also suggested in patients with constipation stemming from opioid use that has been particularly difficult to manage. In addition, in patients with renal impairment, hydromorphone or fentanyl may be preferred alternatives.
The majority of patients with symptomatic bone metastases obtain adequate relief with opioids with optimal pain control usually requiring titration, the use of long-acting opioids, and effective management of breakthrough pain. However, there is a subset of patients who respond poorly for a variety of reasons. For instance, neuropathic pain is often poorly controlled by opioid therapy alone. In addition, in some patients, it is difficult to achieve an acceptable balance between desired analgesia and unwanted side-effects. Clinicians must be attuned to the possibility that opioids, as integral as they are in cancer pain management, may not always work and must be prepared to consider an alternative strategy of pain management.
Alternative approaches to the management of poorly responsive pain include opioid rotation, i.e., switching to a different opioid, aiming to improve the balance between analgesia and undesirable adverse effects, aggressive management of side-effects to allow dose escalation, as well as consideration of non-pharmacologic interventions such as nerve blocks. Another approach that should be considered, particularly in the setting of neuropathic pain, is the use of adjuvant analgesics. Drugs that fall under this class of analgesics include glucocorticoids, analgesic antidepressants (serotonin-norepinephrine reuptake inhibitors and tricyclic antidepressants) and anticonvulsants.
Radiotherapy
Radiation therapy remains a primary therapeutic modality in the management of malignant bone pain, achieving pain reduction in 50–70% of patients and complete pain-relief in up to one-third of patients.
Conventionally fractionated external beam radiation therapy
Several trials have demonstrated that shorter fractionation schedules are as effective as more protracted regimens in the palliation of malignant bone pain although re-treatment as well as fracture rates may be higher with single fraction treatments (9-11). Commonly used dose-fractionation schedules include 8 Gy/1#, 20 Gy/5#, 24 Gy/6#, and 30 Gy/10#. Choice is dictated by factors such as clinician and patient preference, performance status of patient and disease extent.
Stereotactic body radiation therapy (SBRT)
SBRT is characterized by the precise delivery of high radiation doses in a small number of fractions, typically one to five fractions. SBRT has been successfully utilized in several extracranial sites such as the lung, head and neck, liver, pancreas and prostate. It has also been used in skeletal sites and is of particular value in the management of spine metastases.
As previously mentioned, the spine is a frequent site of metastasis in patients with many solid malignancies including lung cancer and is a source of significant morbidity. Common disease manifestations include progressive pain, neurologic deficits, and autonomic dysfunction. Without effective treatment, uncontrolled symptoms inevitably lead to a decline in functional status and a deleterious impact on quality of life. Hence, rapid and sustained pain relief and preservation and/or stabilization of neurologic function are paramount goals in the palliative care of these patients. In addition, with recent advances in systemic therapy and prolongation of life expectancies in this subgroup, durable local tumor control has become an equally imperative end-point.
To date, the mainstay of management for spinal metastases has been conventionally fractionated external beam radiation therapy either as the primary treatment modality or as an adjunct to decompressive surgery. However, radiation doses to this region have traditionally been dictated by the tolerance of the spinal cord and to a lesser extent, that of surrounding normal tissues such as the kidneys and bowel to radiation. In the context of radioresistant tumors, this often means reduction of doses to a level that falls short of optimal therapy.
Foremost among the malignancies historically perceived as being relatively radioresistant are melanoma and renal cell carcinoma. The pathophysiology underlying this apparent intrinsic radioresistance remains poorly defined and is the subject of active research. In vitro measures of cellular recovery suggest that repair of radiation-induced damage is performed with greater proficiency in these histologies than in other cell lines. Amplification of DNA repair genes and increased cellular production of free radical scavengers such as glutathione are among the postulated mechanisms (12,13). Other radiobiologic data derived from irradiated melanoma and renal cancer cell lines indicate that these tumor types have low alpha/beta ratios similar to that of slow-renewal normal tissues, implying that they require higher radiation doses and larger than conventional fraction sizes to achieve effective cytotoxicity (14,15).
For the most part, the available clinical data on melanoma and renal cell carcinoma have been in line with these experimental observations. The response rates of these histologies to conventional radiation have been almost universally poor with most studies reporting rates of pain control and return to ambulation of 30–40% or less (16,17). In addition, documented responses, if any, are characteristically short-lived with a mean duration of symptom control in the order of 2–3 months. To complicate matters further, surgical intervention, when required, to combat the effects of radiation failure is often compromised by significant intraoperative blood loss owing to the vascularity of these tumors, poor visualization and a high rate of peri-operative morbidity (18-20). The end result is a prolongation of recovery times and a delay in the re-institution of vitally needed systemic therapy.
These less than satisfactory outcomes suggest that some form of treatment intensification may be warranted for these radioresistant tumors. Multiple prior reports have described the effectiveness of high-dose single-fraction stereotactic radiosurgery in addressing brain metastases from renal cell carcinoma and melanoma (21-24). Observations of favorable responses to intracranial radiosurgery prompted the initial hypothesis that this dose-escalation approach could be extrapolated to extracranial sites such as the spine.
In addition to its use in radioresistant histologies, SBRT may be considered in patients with a limited number of bone metastases in a limited number of sites. In recent times, the clinical phenomenon of the oligometastatic state is increasingly being recognized. However, there is no real consensus as to the number of metastases and/or disease bulk that constitute the oligometastatic state, with most studies using a cut-off between 3 and 5. The hypothesis is that patients with mono- or oligometastases may have tumors with a more indolent biology, where aggressive ablative treatment such as SBRT directed to the known sites of bone metastases could render patients disease-free for prolonged periods of time. With skeletal oligometastases, in particular those located in the axial skeleton, where disease progression can result in devastating neurologic sequelae as previously discussed, durable local disease control is desirable in this subgroup of patients who are likely to have prolonged survival.
There are two principal technologic innovations that have made SBRT to the spine feasible. The first is the advent of intensity-modulated radiation therapy which has the ability to create steep dose gradients between the tumor and neighboring critical structures with a dose fall-off of approximately 10% per millimeter. This precise dosimetry is desirable in the management of spinal tumors where often, only a few millimeters separate disease from the spinal cord. The second key element has been the development of sophisticated image-guided systems, which serve to minimize treatment errors associated with patient positioning. The synergy of these two technologies has permitted significant reduction of planning target volume (PTV) margins and safe dose escalation with hypofractionation (25).
At present, there is no randomized data comparing conventionally fractionated external beam radiotherapy with SBRT and the use of SBRT is associated with a higher risk of vertebral compression fractures, myelopathy, as well as oesophageal toxicities (26,27). Hence, in clinical practice, SBRT is generally reserved for patients with good performance status with mono-metastatic or oligometastatic bone disease and in patients with vertebral metastases who require re-irradiation. It may also be considered in the management of symptomatic bone metastases arising from relatively radioresistant tumors such as renal cell carcinomas, melanomas and sarcomas.
Radiopharmaceuticals
There is a well-recognized subgroup of patients with widespread bone metastases and consequently, diffuse pain who will not be optimally managed by focal radiotherapy. In such circumstances, bone-targeted radiopharmaceuticals are a viable option with reported response rates between 40% and 90% (28-30). However, the onset of pain relief is generally more gradual compared with conventional radiotherapy, taking up to 8 weeks and they are associated with haematologic toxicities which may be prolonged. Indications for their use include multifocal osteoblastic bone metastases that enhance on radionuclide bone scan and that are refractory to analgesics and life expectancies greater than 3 months. Their use is contraindicated in patients with renal failure, acute spinal cord compression and myelosuppression as well as in pregnant or breastfeeding patients.
Commercially available radioisotopes include samarium-153 and strontium-89 as well as radium-223. Data on the utility of these bone-targeted radioisotopes in the management of symptomatic bone metastases is sparse with the vast majority of literature documenting efficacy of these radiopharmaceuticals coming from patients with metastatic prostrate and breast cancer (28-30).
Surgery
While surgery is generally not the primary modality used in the management of bone metastases, it is increasingly being recognized as an important component of care in certain clinical scenarios, e.g., long bone metastases to prevent impending fractures, to promote osteosynthesis and accelerate bone healing in actual pathologic fractures, to restore patient mobility and function as well as to improve the patient’s overall quality of life. Surgical intervention may also be indicated in spine metastases that are causing mechanical instability or epidural spinal cord or cauda equina compression.
For symptomatic bone metastases involving long bones, there are predictive tools used to assess risk for pathologic fractures. One widely used scoring system to predict fracture risk is the Mirels scoring system where scores are assigned based on lesion site, lesion type, lesion size and degree of pain, with prophylactic fixation recommended for scores greater than or equal to 9 where fracture risk is considered to be 33% or greater (31).
Similarly, in good performance status patients with spine metastases complicated by epidural spinal cord or cauda equina compression and life expectancies exceeding 3 months, a landmark randomized trial demonstrated superior outcomes with decompressive surgery followed by postoperative radiotherapy compared with radiotherapy alone both in terms of preservation of ambulation in ambulatory patients as well as return to ambulation in nonambulatory patients (32).
That said, a careful selection process should be undertaken to avoid futile surgery. Moribund or severely medically ill patients should not be offered operative intervention. In general, in order to achieve meaningful palliation, patients should have an estimated life expectancy that is significantly longer than the time needed to recover from surgery. In this regard, a number of predictive models to estimate prognosis in patients with metastatic malignancy have been developed, the majority of which are specific to patients with vertebral metastases (33), e.g., Tokuhashi scoring system (34,35). These incorporate factors such as extent of skeletal metastases, presence of visceral metastases, primary site of disease, performance status etc. However, most if not all of these models are limited in their ability to accurately predict overall survival.
For patients with long bone or vertebral metastases who have undergone surgical stabilization, postoperative radiation therapy is generally recommended to consolidate the effects of surgery, to promote bone remineralization and healing and reduce the risk of subsequent fractures and loss of fixation by treating residual metastatic disease within the bone.
Systemic therapy
Systemic anticancer treatment, comprising of endocrine therapy, targeted therapies, and conventional chemotherapy, is an important component of care for patients with metastatic bone disease, both in terms of controlling local symptoms and slowing skeletal progression of disease. They contribute to pain relief primarily by reducing tumor bulk but some have been shown to modulate pain signalling pathways as well. However, pain relief is not swiftly achieved and in many instances, particularly in patients with advanced disease with widespread metastases, poor performance status may preclude systemic anticancer treatment. For these reasons, in patients with significant pain arising from bone metastases, local radiation therapy is often undertaken, in combination with optimization of analgesia, prior to consideration of systemic anticancer therapies.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Kam-Weng Fong, Kevin Lee Min Chua) for the series “Radiotherapy in Lung Cancer” published in Journal of Xiangya Medicine. The article has undergone external peer review.
Conflicts of Interest: The author has completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jxym.2018.12.02). The series “Radiotherapy in Lung Cancer” was commissioned by the editorial office without any funding or sponsorship. The author has no other conflicts of interest to declare.
Ethical Statement: The author is accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Lipton A, Fizazi K, Stopeck AT, et al. Superiority of denosumab to zoledronic acid for prevention of skeletal-related events: a combined analysis of 3 pivotal, randomised, phase 3 trials. Eur J Cancer 2012;48:3082-92. [Crossref] [PubMed]
- Martin M, Bell R, Bourgeois H, et al. Bone-related complications and quality of life in advanced breast cancer: results from a randomized phase III trial of denosumab versus zoledronic acid. Clin Cancer Res 2012;18:4841-9. [Crossref] [PubMed]
- Vadhan-Raj S, von Moos R, Fallowfield LJ, et al. Clinical benefit in patients with metastatic bone disease: results of a phase 3 study of denosumab versus zoledronic acid. Ann Oncol 2012;23:3045-51. [Crossref] [PubMed]
- Van Poznak C, Somerfield MR, Barlow WE, et al. Role of Bone-Modifying Agents in Metastatic Breast Cancer: An American Society of Clinical Oncology-Cancer Care Ontario Focused Guideline Update. J Clin Oncol 2017;35:3978-86. [Crossref] [PubMed]
- Mercadante S, Tirelli W, David F, et al. Morphine versus oxycodone in pancreatic cancer pain: a randomized controlled study. Clin J Pain 2010;26:794-7. [Crossref] [PubMed]
- Pigni A, Brunelli C, Caraceni A. The role of hydromorphone in cancer pain treatment: a systematic review. Palliat Med 2011;25:471-7. [Crossref] [PubMed]
- Riley J, Branford R, Droney J, et al. Morphine or oxycodone for cancer-related pain? A randomized, open-label, controlled trial. J Pain Symptom Manage 2015;49:161-72. [Crossref] [PubMed]
- Corli O, Floriani I, Roberto A, et al. Are strong opioids equally effective and safe in the treatment of chronic cancer pain? A multicenter randomized phase IV 'real life' trial on the variability of response to opioids. Ann Oncol 2016;27:1107-15. [Crossref] [PubMed]
- Price P, Hoskin PJ, Easton D, et al. Prospective randomised trial of single and multifraction radiotherapy schedules in the treatment of painful bony metastases. Radiother Oncol 1986;6:247-55. [Crossref] [PubMed]
- Sze WM, Shelley M, Held I, et al. Palliation of metastatic bone pain: single fraction versus multifraction radiotherapy - a systematic review of the randomised trials. Cochrane Database Syst Rev 2004;CD004721. [PubMed]
- 8 Gy single fraction radiotherapy for the treatment of metastatic skeletal pain: randomised comparison with a multifraction schedule over 12 months of patient follow-up. Bone Pain Trial Working Party. Radiother Oncol 1999;52:111-21. [Crossref] [PubMed]
- Fertil B, Malaise EP. Intrinsic radiosensitivity of human cell lines is correlated with radioresponsiveness of human tumors: analysis of 101 published survival curves. Int J Radiat Oncol Biol Phys 1985;11:1699-707. [Crossref] [PubMed]
- Weichselbaum RR, Little JB. Radioresistance in some human tumor cells conferred in vitro by repair of potentially lethal X-ray damage. Radiology 1982;145:511-3. [Crossref] [PubMed]
- Rofstad EK. Radiation biology of malignant melanoma. Acta Radiol Oncol 1986;25:1-10. [Crossref] [PubMed]
- Schwachofer JH, Crooijmans RP, Hoogenhout J, et al. Sublethal damage repair in two radioresistant human tumor cell lines irradiated as multicellular spheroids. Tumour Biol 1991;12:207-16. [Crossref] [PubMed]
- Huguenin PU, Kieser S, Glanzmann C, et al. Radiotherapy for metastatic carcinomas of the kidney or melanomas: an analysis using palliative end points. Int J Radiat Oncol Biol Phys 1998;41:401-5. [Crossref] [PubMed]
- Rades D, Walz J, Stalpers LJ, et al. Short-course radiotherapy (RT) for metastatic spinal cord compression (MSCC) due to renal cell carcinoma: results of a retrospective multi-center study. Eur Urol 2006;49:846-52; discussion 852. [Crossref] [PubMed]
- King GJ, Kostuik JP, McBroom RJ, et al. Surgical management of metastatic renal carcinoma of the spine. Spine (Phila Pa 1976) 1991;16:265-71. [Crossref] [PubMed]
- Broaddus WC, Grady MS, Delashaw JB Jr, et al. Preoperative superselective arteriolar embolization: a new approach to enhance resectability of spinal tumors. Neurosurgery 1990;27:755-9. [Crossref] [PubMed]
- Sundaresan N, Scher H, DiGiacinto GV, et al. Surgical treatment of spinal cord compression in kidney cancer. J Clin Oncol 1986;4:1851-6. [Crossref] [PubMed]
- Brown PD, Brown CA, Pollock BE, et al. Stereotactic radiosurgery for patients with "radioresistant" brain metastases. Neurosurgery 2008;62:790-801. [Crossref] [PubMed]
- Goyal LK, Suh JH, Reddy CA, et al. The role of whole brain radiotherapy and stereotactic radiosurgery on brain metastases from renal cell carcinoma. Int J Radiat Oncol Biol Phys 2000;47:1007-12. [Crossref] [PubMed]
- Mori Y, Kondziolka D, Flickinger JC, et al. Stereotactic radiosurgery for cerebral metastatic melanoma: factors affecting local disease control and survival. Int J Radiat Oncol Biol Phys 1998;42:581-9. [Crossref] [PubMed]
- Mori Y, Kondziolka D, Flickinger JC, et al. Stereotactic radiosurgery for brain metastasis from renal cell carcinoma. Cancer 1998;83:344-53. [Crossref] [PubMed]
- Yamada Y, Lovelock DM, Bilsky MH. A review of image-guided intensity-modulated radiotherapy for spinal tumors. Neurosurgery 2007;61:226-35; discussion 235. [Crossref] [PubMed]
- Rose PS, Laufer I, Boland PJ, et al. Risk of fracture after single fraction image-guided intensity-modulated radiation therapy to spinal metastases. J Clin Oncol 2009;27:5075-9. [Crossref] [PubMed]
- Cox BW, Jackson A, Hunt M, et al. Esophageal toxicity from high-dose, single-fraction paraspinal stereotactic radiosurgery. Int J Radiat Oncol Biol Phys 2012;83:e661-7. [Crossref] [PubMed]
- Coleman R, Aksnes AK, Naume B, et al. A phase IIa, nonrandomized study of radium-223 dichloride in advanced breast cancer patients with bone-dominant disease. Breast Cancer Res Treat 2014;145:411-8. [Crossref] [PubMed]
- Zacho HD, Karthigaseu NN, Fonager RF, et al. Treatment with bone-seeking radionuclides for painful bone metastases in patients with lung cancer: a systematic review. BMJ Support Palliat Care 2017;7:230-7. [PubMed]
- Kolesnikov-Gauthier H, Lemoine N, Tresch-Bruneel E, et al. Efficacy and safety of (153)Sm-EDTMP as treatment of painful bone metastasis: a large single-center study. Support Care Cancer 2018;26:751-8. [Crossref] [PubMed]
- Johnson SK, Knobf MT. Surgical interventions for cancer patients with impending or actual pathologic fractures. Orthop Nurs 2008;27:160-71; quiz 172-3. [Crossref] [PubMed]
- Patchell RA, Tibbs PA, Regine WF, et al. Direct decompressive surgical resection in the treatment of spinal cord compression caused by metastatic cancer: a randomised trial. Lancet 2005;366:643-8. [Crossref] [PubMed]
- Laufer I, Rubin DG, Lis E, et al. The NOMS framework: approach to the treatment of spinal metastatic tumors. Oncologist 2013;18:744-51. [Crossref] [PubMed]
- Tokuhashi Y, Matsuzaki H, Oda H, et al. A revised scoring system for preoperative evaluation of metastatic spine tumor prognosis. Spine (Phila Pa 1976) 2005;30:2186-91. [Crossref] [PubMed]
- Quraishi NA, Manoharan SR, Arealis G, et al. Accuracy of the revised Tokuhashi score in predicting survival in patients with metastatic spinal cord compression (MSCC). Eur Spine J 2013;22:S21-6. [Crossref] [PubMed]
Cite this article as: Thiagarajan A. Management of bone metastases. J Xiangya Med 2019;4:3.