Chest tumors: current trends of clinical practice
Introduction
Radiotherapy (RT) has an important role in the management of lung cancer. Besides requiring detailed pathological assessment, radiation oncologist relies heavily on imaging for diagnosis, staging, treatment planning, monitoring of disease during and after therapy. Advances in RT technology include proton treatment, respiratory gating, immobilization, forward planning, intensity-modulated radiation (IMRT), volumetric-modulated arc therapy (VMAT), tomotherapy, image-guided radiation treatment (IGRT) and on-treatment cone-beam computerized axial tomography scan (CBCT). These allow decreased doses to organ at risk such as brachial plexus, ribs, spinal cord, contralateral esophagus (CE), lung, heart, and adjacent liver.
The literature on RT for chest tumors cancer contains controversies on the exact role and timing of RT in relation to other modalities such as surgery. Relevant literature is selected and presented in this review with practical clinical tips. Mesothelioma and thymoma are presented as an example to illustrate improvements in RT techniques in past decade.
Increasing role of imaging in the treatment of chest tumors
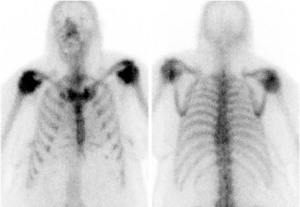
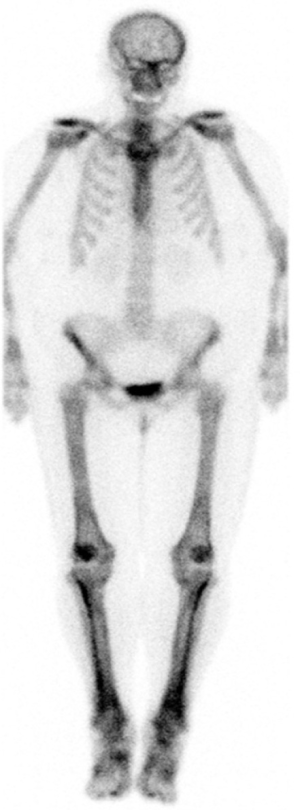
According to the National Comprehensive Cancer Network (NCCN) guideline (1), all patients with non-small cell lung cancer (NSCLC) should undergo staging positron-emission tomography/computerized tomography (PET/CT) scans. Except for peripheral stage IA (T1a,b N0), all patients require brain magnetic resonance imaging (MRI) with gadolinium contrast. Bone scan is no longer mandatory, although it can be ordered if there is clinical suspicion of bone metastasis. It can give extra information as illustrated in Figures 1 and 2.
The maximum standard uptake value (SUVmax) of the primary tumor is correlated with RT treatment outcome (2,3). Researchers found PET scan SUVmax is also correlated with molecular markers. Among Japanese patients with small size NSCLC, the SUVmax was significantly higher in those with programmed death-ligand 1 (PD-L1) expression than in those without (P<0.0001), but no such correlation was found in neuroendocrine tumors (P=0.9638) (4). Another study on Chinese patients demonstrated that SUVmax of primary tumor <7.0, female sex, non-smoker status and adenocarcinoma were predictors of epidermal growth factor receptor (EGFR) mutations in multivariate analysis (5). Anaplastic lymphoma kinase (ALK)-positive patients tended to have a high nodal SUVmax. Younger age and distant metastasis were the only two independent predictors of ALK positivity. When molecular marker study is not available, the clinical factors and SUVmax may help to guide treatment.
PET scan has been used in assisting treatment planning of lung cancer to define the gross tumor from atelectasis (6). It is being investigated as a tool to direct higher radiation dose to a lung tumor, i.e., PET/CT guided RT dose escalation and boost as in “PET BOOST: A randomized phase II trial to assess the efficacy and safety of selective metabolically adaptive radiation dose escalation in locally advanced non-small cell lung cancer receiving definitive chemoRT” (7). In Montefiore Medical Center, researchers employed PET-based dose-painted intensity modulated radiation therapy (IMRT) for stage IIB-III NSCLC with success (8). Tumors or lymph nodes with metabolic tumor volume exceeding 25 cm3 were deemed “high risk” and received 65 Gy/25 fractions (f). Smaller lesions were treated with 57 Gy or 52.5 Gy/25 f (after November 2014). Patients also received concurrent weekly carboplatin (area under the curve =2) and paclitaxel (45 mg/m2).
During treatment, researchers can perform 18F-fluorothymidine (FLT) PET and 18F-fluorodeoxyglucose (FDG) PET scans on patients with NSCLC to assess response and prognosis (9). Locoregional progression during radiation therapy was observed in 5 (8%) patients, prompting larger radiation therapy fields. In addition, distant metastases detected in PET scans during treatment would change treatment intent from curative to palliative.
To illustrate the importance of on-treatment monitoring, we treated a 70-year-old patient whose pretreatment CT images demonstrated mediastinal nodal involvement only. PET scan was not part of our routine pretreatment investigations 20 years ago. We decided to treat her with chemo-radiation (CRT). After the first cycle of chemotherapy, a right upper lobe primary showed up, likely as a result of “tumoritis”. This was included in the RT field. She was cured without radiation pneumonitis and is still alive with no evidence of disease recurrence at present. Similarly, when changes are seen in onboard on-treatment monitoring system with kilovoltage imaging or cone beam CT, we would immediately re-plan the treatment.
The standard post-treatment follow-up for lung cancer includes CT scan chest with or without contrast. However, PET/CT scan may be used to monitor disease after treatment, with a plan for boost stereotactic RT to any residual tumor, or salvage chemotherapy. After stereotactic RT, the primary tumor is obscured by post-radiation changes. PET scan can pick up metabolically active tumor from ground-glass interstitial changes, fibrosis or atelectasis due to RT. This will also facilitate biopsy to target the residual or new tumor focus.
Stereotactic body RT (SBRT)
In the past decade, SBRT was increasingly used to escalate radiation dose to small primary tumors only without nodal coverage. The use of SBRT in management of early stage NSCLC has been described in detail, in part, by the Cancer Care Ontario treatment guideline and readers are encouraged to review it for further information (10). Following SBRT, radiologists and radiation oncologists may interpret radiologic changes differently. A synoptic scale was proposed by the Princess Margaret Hospital in Toronto (11) hoping to increase agreement on reporting.
Another complication of SBRT is the potential association with rib fracture which is significantly associated with dose to 0.5 cc of the ribs (D0.5), and the volume of the rib receiving at least 25 Gy (V25) (12). Interestingly, 25 Gy in 3 treatments is equivalent to 50 Gy given at 2 Gy/f, using an alpha-beta ratio of 4 in linear-quadratic equation.
Case selection for local RT +/– chemotherapy
Pulmonary function test (PFT) requirements for conventionally fractionated RT are: the best pre- or post-bronchodilator forced expiratory volume in 1 sec (FEV1) ≥1.2 litres/sec, or ≥50% predicted in the Radiation Therapy Oncology Group (RTOG) 0617 study (13). Our experience of treating patients with FEV1 ≥1 results in moderately severe respiratory complications (especially if pretreatment FEV1 value is close to 1). Patients become short of breath but often not yet qualify for government-sponsored oxygen supply. An association between radiation pneumonitis risk and dose volume histogram (DVH) parameters has been described. Recommendations for reporting and conduct of further research into association between DVH metrics and pneumonitis risk have been provided (14). Respecting lung organ radiation tolerance, careful monitoring pulmonary functions pre- and post-therapy is essential.
Fortunately, the newer SBRT is not often limited by poor baseline pulmonary function and allows radical treatment for inoperable early stage lung cancer. In RTOG 0236 with 18 Gy ×3 treatments, at follow up at 2 years, the mean percentage predicted FEV1 and diffusing capacity for carbon monoxide (DLCO) declines were 5.8% and 6.3%, respectively, with minimal changes in arterial blood gases and no significant decline in oxygen saturation (15). Baseline PFT was not predictive of any pulmonary toxicity following SBRT. Whole-lung V5 (the percentage of normal lung tissue receiving 5 Gy), V10, V20, and mean dose to the whole lung were almost identical between patients who developed pneumonitis and patients who were pneumonitis-free. Still in clinical practice, some radiation oncologists prefer to have at least a FEV1 of 0.7 before SBRT, to be on the safe side.
In the literature, solitary peripheral cancer of ≤4 cm is selected for SBRT. Larger primary tumor has lower local control, more regional/distant metastasis and increased radiation toxicity. The Princess Margaret Hospital found that up to 5.7 cm in tumor diameter or 100 cc in tumor volume can be treated and suggested adjuvant therapy may be considered for optimal cancer control (16).
For many years, conventional RT 60 Gy/30 f alone was the standard treatment for patients with locoregionally advanced NSCLC, despite a 5-year survival rate of only 3–20% following such therapy. Sequential chemotherapy and RT was studied in the early days. From May 1984 through May 1987, the landmark study of Dillman (17), cancer and leukemia group B (CALGB) 8433 trial randomized patients with stage III NSCLC with Eastern Cooperative Oncology Group (ECOG) with good performance status to two treatment arms. Group I received cisplatin and vinblastine followed by radiation therapy with 60 Gy given in 30 fractions beginning on day 50 (CT-RT group). Group 2 received radiation therapy with 60 Gy alone beginning on day 1 (RT group). At 3 years of follow up, group 1 had a higher incidence of serious infections requiring hospitalization (7 vs. 3 percent in group 2) and severe weight loss (14 vs. 6 percent), but there were no treatment-related deaths. After more than 7 years of follow-up, the median survival remains greater for the CT-RT group (13.7 months) than for the RT group (9.6 months) (P=0.012, log-rank test, two-sided) (18). The percentages of patients surviving after years 1 through 7 were 54, 26, 24, 19, 17, 13, and 13 for the CT-RT group and 40, 13, 10, 7, 6, 6, and 6 for the RT group.
Similarly, the intergroup study showed chemotherapy followed by radiation therapy (60 Gy/30 f) resulted in superior survival to either hyperfractionated radiation (69.6 Gy/58 f at 1.2 Gy/f twice a day) or standard radiation (60 Gy/30 f) in surgically unresectable stage II–III NSCLC (19). Nowadays, patients with locally advanced lung cancer should be treated with concurrent RT and cisplatin-based chemotherapy per RTOG 9410 study (20). A primary tumor size ≤6 cm has a better outcome according to our local experience (21).
For the elderly ≥70 years old with stage III NSCLC, meta-analysis had shown that overall survival (OS) [hazard ratio (HR), 0.66, 95% CI 0.53–0.82; 3 trials; 407 patients] and progression-free survival (PFS) (HR, 0.67, 95% CI 0.53–0.85; 2 trials; 327 patients) both favored CRT compared to RT alone (22). Risk of treatment-related death and grade 3+ pneumonitis were not significantly different between the younger and older age groups. With the exception of increased hematological toxicity, CRT appears to be tolerable in fit elderly patients and represents a reasonable standard of clinical care.
Although concurrent CRT is strongly recommended, sequential CRT or RT alone is appropriate for frail patients who are unable to tolerate concurrent therapy per NCCN guidelines. Another indication for sequential CRT is a large tumor volume, when shrinkage by chemotherapy first would allow satisfactory RT planning within tolerance of organs at risk.
Role of RT before and after surgery
Advantage of trimodality over CRT treatment is still controversial. Albain et al. studied 396 patients with stage T1–3pN2M0 NSCLC (23). After concurrent induction chemotherapy (two cycles of cisplatin and etoposide) plus RT (45 Gy) were given. If no progression, patients are randomized to group 1 of resection and group 2 of continued RT uninterrupted up to 61 Gy. Two additional cycles of cisplatin and etoposide were given in both groups. OS benefit was seen for those who underwent lobectomy but not pneumonectomy. Those with mediastinal nodal clearance (MNC) benefit from the surgery. Importance of MNC was also found in the phase II SWOG 8805 study and RTOG 0229 with 38% and 63% MNC rates respectively (24,25).
Preoperative CRT is used to increase chance of resection, e.g., for superior sulcus tumors. Preoperative chemotherapy followed by postoperative RT and/or chemotherapy is another feasible sequence for locally advanced NSCLC. Oncologists may ask what is the role of preoperative RT? Does it add to preoperative chemotherapy? A meta-analysis published in 2012 of phase II, III studies and retrospective studies found no significant survival benefit of preoperative CRT to chemotherapy for stage IIIA (N2) disease (26). In the National Cancer Database, 1,076 patients treated between 2003 to 2005, the 5-year OS was 39.2% for neoadjuvant CRT vs. 38.6% for neoadjuvant chemotherapy [P= non-significant (NS)] on multivariable regression (27). However neoadjuvant CRT had significantly less risk of residual nodal disease and adverse pathological features (P=0.0023).
A more recent cohort study on 138 stage III (N0–2) patients found that the median OS was significantly higher after trimodality therapy than after CRT (81 versus 31.8 months, P=0.0068) (28). This benefit was restricted to nodal pathologic complete response (N-PCR) (n=50, 83.2 versus 31.8 months, P=0.0004), as residual nodal disease (n=19) experienced poor OS (16.1 months). On multivariable analyses, N-PCR had superior OS (HR, 0.38; P=0.0012), PFS (HR, 0.42; P=0.0005), and distant metastasis free survival (HR, 0.42; P=0.0007) compared with CRT.
Another controversial question is the timing of postoperative RT (PORT). Using the National Cancer Database, after lobectomy for pN2 with negative margin (R0), postoperative adjuvant chemotherapy followed by RT (C→PORT is significantly better than postoperative concurrent CRT, 58.8 vs. 40.4 months (log-rank P<0.001) (29). For the cohort with R1–2, the median OS was 42.6 months for patients who received C→PORT versus 38.5 months for patients who received CRT (log-rank P=0.42). Similarly another study on the same database, 703 patients had received PORT <8 weeks and 926 had received PORT ≥8 weeks postoperatively. The receipt of PORT after 8 weeks was associated with better OS (P=0.0044) (30). No significant differences were found in survival in the concurrent group comparing early and later time to relapse (P=0.91). Our interpretation is a theoretically better delivery of chemotherapy before RT which causes endarteritis obliterans (blocking of small vessels by fibrosis). In addition, earlier use of adequate dose of chemotherapy helps to eliminate microscopic disease. After RT, general condition of patients and tolerance to normal dose of chemotherapy may be affected. All these factors potentially enhance the effect of CRT on disease control. For those with residual disease after surgery, there may be a bias to give RT early concurrently with chemotherapy if the residual is large resulting in poorer outcome of early PORT.
Sparing of organs at risk
In our experience, the esophagus is easily visible in planning CT in only some levels. Oral contrast such as Esopho-cat, 30 gm barium sulfate cream or water can outline esophagus better to facilitate radiation treatment planning. Researchers at the Massachusetts General Hospital designed a way to spare the CE with intensity modulated RT (IMRT) (31) when the gross tumor is within 1 cm of esophagus. The CE was an avoidance structure, and contoured at least 5 mm away from the gross tumor. This can allow dose fall off by IMRT technique. The internal target volume (ITV) can be well covered by 66.6 Gy while the CE receives 45 Gy. They proposed CE dose constraints of V45 <2.5 cc and V55 <0.5 cc.
At the London Regional Cancer Program (LRCP), a randomized phase III study—PROACTIVE (Palliative radiation of advanced central lung tumors with intentional avoidance of the esophagus) is launched to ascertain the feasibility of organ at risk sparing (7).
Healthy lung can be spared by focusing the radiation beam flow onto the emphysematous regions. The Myrian© software based on diagnostic CT (DCT) extracted the data of emphysema and transferred to the RT treatment planning system (32). Patients were then treated with helical tomotherapy.
The other critical structure immediately adjacent to lung and susceptible to radiation effect is the heart. Recently researchers develop an interest on heart doses. Doses to different sub-volumes of heart were analyzed by Wang et al. for pericardial (symptomatic effusion and pericarditis), ischemia (myocardial infarction and unstable angina), and arrhythmia (33). The stereotactic ablative RT program of the Princess Margaret Hospital found the maximum dose to bilateral ventricles to be significant for OS on multivariate analysis (34). In Manchester United Kingdom, analysis of 1,101 patients quantified that greater than 8.5 Gy to the base of heart had worse survival (log-rank P<0.001, HR, 1.2) (35). Further study on the effect of radiation to the heart will soon begin at the LRCP to identify acute radiation-induced cardiac disease after NSCLC radiation therapy with advanced multi-modality imaging.
Mesothelioma and thymoma as illustrative examples
To illustrate the above discussion, selection criteria for RT in mesothelioma according to the NCCN guidelines are: ECOG performance status ≤1, good pulmonary function, adequate function of contralateral kidney confirmed by renal scan, absence of disease in abdomen, contralateral chest or elsewhere, and the patient does not require supplemental oxygen.
RT for mesothelioma is difficult due to a large target volume of drain sites, nodal stations, entire ipsilateral hemithorax, and multiple critical structures. Dose inhomogeneity is common and is associated with local recurrence (36,37). Allen et al. recommended decreasing the number of beams on the superior portion of planning target volume (38). The NCCN guidelines recommends the mean lung dose be kept as low as possible, preferably <8.5 Gy.
What is the optimal RT dose for mesothelioma? A total dose of 45 Gy was associated with a 46% local and/or distant failure rate after median follow-up of 9.5 months. On the other hand, a higher dose of 54 Gy resulted in 46% risk of radiation pneumonitis (39). Therefore a reasonable dose appears to be 50 Gy/25 f to entire hemithorax including chest wall incisions & drain sites excluding intact lung with simultaneous integrated boost to FDG avid areas to 60 Gy by tomotherapy (40).
The Surgery for Mesothelioma After Radiation Therapy (SMART) phase I/II trial used a short hypofractionated RT regimen of 25 Gy in five daily fractions during 1 week to the entire ipsilateral hemithorax with concomitant 5 Gy boost to areas at risk followed by extrapleural pneumonectomy within 1 week of RT completion (41). Adjuvant chemotherapy was offered to ypN2 patients on final pathologic findings.
Useful RT techniques for treatment of thoracic tumors like mesothelioma or thymoma include: IMRT, VMAT, IGRT, tomotherapy (Figure 3) (42), and proton treatment. Proton treatment has a slight improvement in target coverage. It has clear advantages in dose conformity and homogeneity and can avoid many critical organs in thorax and abdomen. Intensity-modulated proton therapy (IMPT) can lower doses to contralateral lung, heart, esophagus, liver, ipsilateral kidney, especially when a mediastinal boost is required for nodal disease. Disadvantages of proton treatment are: more uncertainty due to motion, higher cost and less accessibility due to limited number of proton centers. However, the cost is counterbalanced by reduction of side effects & toxicity-related hospitalization (43). The cost is decreasing over time due to reduced prices for the building, machine, maintenance, overhead, and newer, shorter treatment programs. After extrapulmonary pneumonectomy for mesothelioma, air cavities are common (range: 0–850 cc), decreasing from 0 to 18.5 cc/day. Proton therapy dose distributions are more susceptible to changing air cavities, emphasizing the need for adaptive RT and re-planning (44,45).
Lastly, we strongly encourage multidisciplinary care, thorough quality-assurance of RT treatment, and the use of reporting guideline in patient notes, as shown in Figure 4. A short end of RT summary is not helpful for future management in case relapse occurs.

Conclusions
New technologies for thoracic tumor treatment greatly improve radiation therapy planning and delivery. Together with multidisciplinary care, and careful selection of appropriate patients, we can increase the therapeutic ratio, with better tolerance of treatment and achieving higher doses without significant side effects on critical organs at risk. Stereotactic body RT has translated to better local control, particularly in patients with multiple comorbidities previously not amendable for any treatment intervention. The potential impact of more advances in RT on survival awaits further results from ongoing investigations.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Kam-Weng Fong, Kevin Lee Min Chua) for the series “Radiotherapy in Lung Cancer” published in Journal of Xiangya Medicine. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jxym.2018.07.02). The series “Radiotherapy in Lung Cancer” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Disclaimer: Presented in part as a workshop in the Annual Scientific Meeting of Canadian Association of Radiation Oncology, September 13–16, 2017 in Toronto, Canada.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- NCCN clinical practice guidelines in oncology (NCCN guidelines). Non-small cell lung cancer. Version 5.2018 [published 27 June 2018, accessed 13 July 2018]. Available online: https://www.nccn.org/professionals/physician_gls/default.aspx#all
- Zhu D, Wang Y, Wang L, et al. Prognostic value of the maximum standardized uptake value of pre-treatment primary lesions in small-cell lung cancer on 18F-FDG PET/CT: a meta-analysis. Acta Radiol 2017; [Epub ahead of print]. [Crossref] [PubMed]
- Kocher MR, Sharma A, Garrett-Mayer E, et al. Pretreatment 18F-fluorodeoxyglucose positron emission tomography standardized uptake values and tumor size in medically inoperable non small cell lung cancer is prognostic of overall 2-year survival after stereotactic body radiation therapy. J Comput Assist Tomogr 2018;42:146-50. [PubMed]
- Takada K, Toyokawa G, Tagawa T, et al. Association between PD-L1 expression and metabolic activity on (18)F-FDG PET/CT in patients with small-sized lung cancer. Anticancer Res 2017;37:7073-82. [PubMed]
- Lv Z, Fan J, Xu J, et al. Value of (18)F-FDG PET/CT for predicting EGFR mutations and positive ALK expression in patients with non-small cell lung cancer: a retrospective analysis of 849 Chinese patients. Eur J Nucl Med Mol Imaging 2018;45:735-50. [Crossref] [PubMed]
- Ung YC, Bezjak A, Coakley, et al. Positron emission tomography in radiation treatment planning for lung cancer. Cancer Care Ontario lung cancer guideline #7-18. Available online: https://www.cancercareontario.ca/en/guidelines-advice/types-of-cancer/811
- Clinical Trials. Available online: http://www.lhsc.on.ca/Research_Training/LRCP/Clinical_Trials/trials.htm?query_id=52
- Ohri N, Bodner WR, Kabarriti R, et al. Positron emission tomography-adjusted intensity modulated radiation therapy for locally advanced non-small cell lung cancer. Int J Radiat Oncol Biol Phys 2017; [Epub ahead of print]. [PubMed]
- Everitt S, Ball D, Hicks RJ, et al. Prospective study of serial imaging comparing fluorodeoxyglucose positron emission tomography (PET) and fluorothymidine PET during radical chemoradiation for non-small cell lung cancer: reduction of detectable proliferation associated with worse survival. Int J Radiat Oncol Biol Phys 2017;99:947-55. [Crossref] [PubMed]
- Falkson CB, Vella ET, Yu E, et al. Guideline for RT with curative intent in patients with early-stage medically inoperable non-small-cell lung cancer. Curr Oncol 2017;24:e44-9. [Crossref] [PubMed]
- Raziee H, Hope A, Faruqi S, et al. Classification and reporting of late radiographic changes after lung stereotactic body RT: proposing a new system. Clin Lung Cancer 2015;16:e245-51. [Crossref] [PubMed]
- Taremi M, Hope A, Lindsay P, et al. Predictors of RT induced bone injury (RIBI) after stereotactic lung RT. Radiat Oncol 2012;7:159. [Crossref] [PubMed]
- RTOG-0617: A randomized phase III comparison of standard-dose (60 Gy) versus high-dose (74 Gy) conformal radiotherapy with concurrent and consolidation Carboplatin/Paclitaxel +/- Cetuximab (IND #103444) in patients with stage IIIA/IIIB non-small cell lung cancer [last updated 26 October 2011, assessed 26 Nov 2017]. Available online: https://www.nrgoncology.org/Clinical-Trials/Protocol-Table
- Rodrigues G, Lock M, D'Souza D, et al. Prediction of radiation pneumonitis by dose - volume histogram parameters in lung cancer--a systematic review. Radiother Oncol 2004;71:127-38. [Crossref] [PubMed]
- Stanic S, Paulus R, Timmerman RD, et al. No clinically significant changes in pulmonary function following stereotactic body radiation therapy for early- stage peripheral non-small cell lung cancer: an analysis of RTOG 0236. Int J Radiat Oncol Biol Phys 2014;88:1092-9. [Crossref] [PubMed]
- Allibhai Z, Taremi M, Bezjak A, et al. The impact of tumor size on outcomes after stereotactic body radiation therapy for medically inoperable early-stage non-small cell lung cancer. Int J Radiat Oncol Biol Phys 2013;87:1064-70. [Crossref] [PubMed]
- Dillman RO, Seagren SL, Propert KJ, et al. A randomized trial of induction chemotherapy plus high-dose radiation versus radiation alone in stage III non-small-cell lung cancer. N Engl J Med 1990;323:940-5. [Crossref] [PubMed]
- Dillman RO, Herndon J, Seagren SL, et al. Improved survival in stage III non-small-cell lung cancer: seven-year follow-up of cancer and leukemia group B (CALGB) 8433 trial. J Natl Cancer Inst 1996;88:1210-5. [Crossref] [PubMed]
- Sause W, Kolesar P, Taylor S IV, et al. Final results of phase III trial in regionally advanced unresectable non-small cell lung cancer: Radiation Therapy Oncology Group, Eastern Cooperative Oncology Group, and Southwest Oncology Group. Chest 2000;117:358-64. [Crossref] [PubMed]
- Curran WJ Jr, Paulus R, Langer CJ, et al. Sequential vs. concurrent chemoradiation for stage III non-small cell lung cancer: randomized phase III trial RTOG 9410. J Natl Cancer Inst 2011;103:1452-60. [Crossref] [PubMed]
- Yu E, Tai P, Ash R, et al. Definitive radiation therapy management of medically non-resectable clinically localized non-small cell lung cancer: results and prognostic factors. Nowotwory 2007;57:263-9.
- Dawe DE, Christiansen D, Swaminath A, et al. ChemoRT versus RT alone in elderly patients with stage III non-small cell lung cancer: A systematic review and meta-analysis. Lung Cancer 2016;99:180-5. [Crossref] [PubMed]
- Albain KS, Swann RS, Rusch VW, et al. RT plus chemotherapy with or without surgical resection for stage III non-small-cell lung cancer: a phase III randomised controlled trial. Lancet 2009;374:379-86. [Crossref] [PubMed]
- Albain KS, Rusch VW, Crowley JJ, et al. Concurrent cisplatin/etoposide plus chest RT followed by surgery for stages IIIA (N2) and IIIB non-small-cell lung cancer: mature results of Southwest Oncology Group phase II study 8805. J Clin Oncol 1995;13:1880-92. [Crossref] [PubMed]
- Suntharalingam M, Paulus R, Edelman MJ, et al. Radiation therapy oncology group protocol 02-29: a phase II trial of neoadjuvant therapy with concurrent chemotherapy and full-dose radiation therapy followed by surgical resection and consolidative therapy for locally advanced non-small cell carcinoma of the lung. Int J Radiat Oncol Biol Phys 2012;84:456-63. [Crossref] [PubMed]
- Shah AA, Berry MF, Tzao C, et al. Induction chemoradiation is not superior to induction chemotherapy alone in stage IIIA lung cancer. Ann Thorac Surg 2012;93:1807-12. [Crossref] [PubMed]
- Sher DJ, Fidler MJ, Liptay MJ, et al. Comparative effectiveness of neoadjuvant chemoRT versus chemotherapy alone followed by surgery for patients with stage IIIA non-small cell lung cancer. Lung Cancer 2015;88:267-74. [Crossref] [PubMed]
- Ziel E, Hermann G, Sen N, et al. Survival benefit of surgery after chemoRT for stage III (N0-2) non-small-cell lung cancer is dependent on pathologic nodal response. J Thorac Oncol 2015;10:1475-80. [Crossref] [PubMed]
- Francis S, Orton A, Stoddard G, et al. Sequencing of postoperative RT and chemotherapy for locally advanced or incompletely resected non-small-cell lung cancer. J Clin Oncol 2018;36:333-41. [Crossref] [PubMed]
- Sura K, Grills IS, Vu CC, et al. Improved survival with increased time-to-radiation and sequential chemotherapy after surgery for pN2 non-small-cell lung cancer. Clin Lung Cancer 2018;19:e185-94. [Crossref] [PubMed]
- Al-Halabi H, Paetzold P, Sharp GC, et al. A contralateral esophagus-sparing technique to limit severe esophagitis associated with concurrent high-dose radiation and chemotherapy in patients with thoracic malignancies. Int J Radiat Oncol Biol Phys 2015;92:803-10. [Crossref] [PubMed]
- Jumeau R, Peguret N, de Bari B, et al. Sparing healthy lung by focusing the radiation beam flow onto the emphysematous regions in the treatment of lung cancer. J Med Imaging Radiat Oncol 2017;61:252-7. [Crossref] [PubMed]
- Wang K, Pearlstein KA, Patchett ND, et al. Heart dosimetric analysis of three types of cardiac toxicity in patients treated on dose-escalation trials for Stage III non-small-cell lung cancer. Radiother Oncol 2017;125:293-300. [Crossref] [PubMed]
- Wong OY, Yau V, Kang J, et al. Survival impact of cardiac dose following lung stereotactic body RT. Clin Lung Cancer 2018;19:e241-6. [Crossref] [PubMed]
- McWilliam A, Kennedy J, Hodgson C, et al. Radiation dose to heart base linked with poorer survival in lung cancer patients. Eur J Cancer 2017;85:106-13. [Crossref] [PubMed]
- Tai P, Yu E, Assouline A, Joseph K. Mesothelioma – epidemiology and management. Curr Resp Med Review 2011;7:364-76. [Crossref]
- Tai P, Joseph K, Assouline A, et al. Mesothelioma. Curr Resp Review 2014;10:206-20.
- Allen AM, Schofield D, Hacker F, et al. Restricted field IMRT dramatically enhances IMRT planning for mesothelioma. Int J Radiat Oncol Biol Phys 2007;69:1587-92. [Crossref] [PubMed]
- Miles EF, Larrier NA, Kelsey CR, et al. Intensity-modulated RT for resected mesothelioma: the Duke experience. Int J Radiat Oncol Biol Phys 2008;71:1143-50. [Crossref] [PubMed]
- Minatel E, Trovo M, Polesel J, et al. Tomotherapy after pleurectomy/decortication or biopsy for malignant pleural mesothelioma allows the delivery of high dose of radiation in patients with intact lung. J Thorac Oncol 2012;7:1862-6. [Crossref] [PubMed]
- Cho BC, Feld R, Leighl N, et al. A feasibility study evaluating Surgery for Mesothelioma After Radiation Therapy: he "SMART" approach for resectable malignant pleural mesothelioma. J Thorac Oncol 2014;9:397-402. [Crossref] [PubMed]
- Minatel E, Trovo M, Polesel J, et al. Radical pleurectomy/decortication followed by high dose of radiation therapy for malignant pleural mesothelioma. Final results with long-term follow-up. Lung Cancer 2014;83:78-82. [Crossref] [PubMed]
- Chang JY, Jabbour SK, De Ruysscher D, et al. Consensus statement on proton therapy in early-stage and locally advanced non-small cell lung cancer. Int J Radiat Oncol Biol Phys 2016;95:505-16. [Crossref] [PubMed]
- Krayenbuehl J, Hartmann M, Lomax AJ, et al. Proton therapy for malignant pleural mesothelioma after extrapleural pleuropneumonectomy. Int J Radiat Oncol Biol Phys 2010;78:628-34. [Crossref] [PubMed]
- Pan HY, Jiang S, Sutton J, et al. Early experience with intensity modulated proton therapy for lung-intact mesothelioma: A case series. Pract Radiat Oncol 2015;5:e345-53. [Crossref] [PubMed]
Cite this article as: Tai P, Falkson C, Yu E. Chest tumors: current trends of clinical practice. J Xiangya Med 2018;3:28.