Surgery versus stereotactic body radiotherapy in medically operable non-small cell lung cancer
Introduction
The current gold standard for treatment of early stage non-small cell lung cancer (NSCLC) is surgical resection by means of a lobectomy, which is the surgical removal of an entire lobe of the lung, together with systematic hilar and mediastinal lymph node evaluation (1). A lung cancer patient is considered medically operable if a thoracic surgeon has judged that the patient can safely undergo surgical resection for the lung cancer with acceptable risk. A variety of parameters, including age, cardiopulmonary function, co-morbidities and performance status, have been used as a guide to determine fitness for surgery (2,3). However, it is ultimately a subjective decision, as a more experienced (or less risk averse) surgeon may consider a marginal patient operable, whereas a less experienced (or more risk averse) surgeon may decide otherwise. Operability is also a moving target, as various advances in surgical technique may change our perspective of operability in the future.
Current evidence shows that surgical resection offers a 5-year survival rate up to 92% for stage I lung cancer (4). Surgery also provides patients and their doctors with an opportunity to properly stage the cancer through histopathology analysis of the tumor tissue and surrounding lymph nodes, which in turn allows for appropriate adjuvant treatment to be administered in a timely fashion for patients who would benefit from it. However, surgical resection is necessarily invasive, carries surgical risk, and also requires a period of recovery for the patient. As a result, there is strong interest in less invasive methods of treatment.
Stereotactic body radiotherapy (SBRT), also known as stereotactic ablative radiotherapy (SABR), was first developed in the 1990’s, based on previous work involving stereotactic radiosurgery in the brain that was developed in the 1950’s (5-7). SBRT was adapted to treat tumors throughout the body, including NSCLC in the past decade (8-10). SBRT is an improvement over conventional radiotherapy as it delivers higher doses of radiation targeted to a smaller area, and is widely used today to treat surgically inoperable NSCLC (11-14). Complications arising from SBRT in the immediate aftermath of treatment tend to be better tolerated as compared to surgical complications (15,16), and as a result, there is now interest in exploring whether the use of SBRT can be expanded to operable early stage NSCLC patients.
In this review, we will consider the relative advantages of SBRT over surgery, and vice versa in the treatment of NSCLC. Next, we will review the available literature comparing the two modalities for treatment of medically operable NSCLC patients. Finally, we will look at the future direction of surgery in the treatment of NSCLC, and whether newer surgical techniques such as video-assisted thoracoscopic surgery (VATS) can eliminate some of the disadvantages of surgery and improve patient outcome, as well as comment on avenues of future research.
Benefits of SBRT over surgery in the treatment of lung cancer
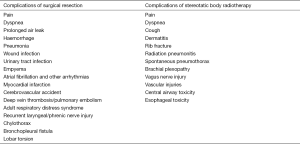
The major benefit of SBRT over surgery is that it is an outpatient and non-invasive technique to treat lung cancer. In contrast, although lobectomy is the standard treatment for early stage NSCLC, it is traditionally done as an open operation that requires general anesthesia and inpatient hospitalization. Moreover, open lobectomy requires an extended period of recovery for the patient, and can result in various complications such as the development of a prolonged air leak [which occurs in 7–18% of cases, although it usually only results in a delay in discharge from hospital (17-20)], hemorrhage, infection (pneumonia being the most common), respiratory failure, atrial fibrillation and other cardiovascular events (21,22). Other rarer complications include nerve injury, chylothorax, bronchopleural fistula and lobar torsion (21,22) (Figure 1).
Initial results from the use of SBRT to treat early stage NSCLC have established that it is a well-tolerated procedure with low rates of complications, even in patients deemed too high risk for surgery (23,24). Serious complications from SBRT are rare, but include central airway toxicity, esophageal toxicity, vascular injuries, spontaneous pneumothorax, radiation pneumonitis, chest wall toxicity, brachial plexopathy and vagus nerve injury (16) (Figure 1). However, the incidence of these complications varies depending on the location of the tumor, with SBRT treatment of central tumors—those within 2 cm of the proximal bronchial tree—causing excessive toxicity (16,25).
Benefits of surgery over SBRT in the treatment of lung cancer
Adequacy of treatment
By surgically removing the lung tumor, a histopathology analysis of the specimen can be carried out, which allows for the determination of whether an R0 resection (during which all disease, both gross and microscopic is removed) was achieved. Since it has been demonstrated that patients with an R0 resection have better outcomes compared to patients in which there is residual microscopic (R1) or gross (R2) disease left behind (26,27), an R0 resection is generally desired. In the event that subsequent histopathology analysis reveals that only an R1 or R2 resection was achieved, this knowledge affords an early opportunity soon after surgery to discuss possible further resection or adjuvant treatment in order to decrease the odds of recurrence and improve long-term survival (28). With SBRT, determination of local control is entirely dependent on follow-up imaging, which can be complicated to interpret in light of the treatment effect from SBRT, leading to potential delays in assessing the adequacy of local treatment and need for salvage therapy (29,30).
In higher risk patients, a sublobar resection instead of a formal lobectomy may be offered to better preserve lung function post-surgery, and hence decrease the risk of surgery (31). Sublobar resections (e.g., segmentectomies or wedge resections) are smaller operations with less lung parenchyma removed, and are better tolerated with lower rates of major complications, even in high risk patients (31,32). Moreover, there is reason to believe that a sublobar resection may give comparable results to a lobectomy in certain subgroups of early stage NSCLC (33,34). Furthermore, even though a sublobar resection may compromise on the extent of margins and local control, information on the adequacy of margins is still obtained and the surrounding lymph nodes are still removed, thus offering important advantages over SBRT (35,36).
Primary tumour analysis
In patients with a lung lesion in whom the histopathological diagnosis of NSCLC has yet to be established, surgical resection is sometimes performed in order to obtain a diagnosis of the lung lesion, in addition to potentially performing a therapeutic procedure in the event of a malignancy. This is not an uncommon situation, as some lesions are not amenable to bronchoscopic or percutaneous biopsy easily, while others have had a biopsy procedure but the yield was non-diagnostic. Some patients may also opt for upfront surgery if they are not keen for a separate diagnostic procedure prior to surgery. In the event that the lesion removed is found to be benign, these patients do not require further adjuvant treatment or the close subsequent follow-up that NSCLC patients require, and their care can be managed appropriately (37-39). By offering SBRT (and the attendant need for close radiological follow-up for a significant period of time) with no tissue removed, we are doing these patients with benign disease a disservice.
In an analysis of solitary pulmonary nodules >20 mm on CT scan, only 64–82% were actually found to be malignant (40). Even among lesions with significant FDG uptake on PET-CT, only 85% were found to be malignant (41). It has been shown that SBRT treatment of lung tumors without histological proof is associated with better survival, compared to SBRT treatment of histologically diagnosed lung cancer. This suggests that a not insignificant proportion of the lung lesions being treated with SBRT without histological proof is actually benign disease (42). Therefore, we need to be circumspect in interpreting outcome data from SBRT studies which include patients who have been treated with SBRT without a prior histopathological diagnosis of NSCLC (43-47).
A histopathological analysis of the removed primary tumor can also offer valuable information that will affect subsequent treatment. It has been reported that a sizable proportion of patients with clinical T1 on preoperative imaging are ultimately found to have pathological T2 or above after resection (48,49). This has implications for adjuvant chemotherapy, as it has been shown that adjuvant chemotherapy confers a significant benefit in patients with completely resected NSCLC larger than 4 cm (50). In addition, clinical understaging of T status may result in a smaller radiation treatment zone if these patients were treated with SBRT, leading to decreased efficacy of SBRT (49).
Furthermore, a removed tumor can be examined for breaching of the pleura, the presence of angiolymphatic invasion, and other histological features which are associated with poorer prognosis. The presence of any of these can aid the oncologist in deciding how strongly to push for the patient to continue with adjuvant chemotherapy (51,52). In an analysis of propensity-matched NSCLC patients from the National Cancer Database who underwent surgery or received SBRT, there was a survival advantage for patients who underwent surgery, and at least part of this may be due to the significantly higher utilization of adjuvant chemotherapy in the surgical arm (53). Therefore, by removing the entire tumor and surrounding tissue surgically, we can glean pertinent information that is crucial for determining subsequent follow-up and treatment - information that is lost when we attempt to treat lung cancer in situ using SBRT.
Lymph nodes resection and analysis
Another major benefit of surgery is the removal of surrounding lymph nodes at the time of surgery, which provides potential therapeutic and diagnostic advantages over SBRT. In the case of patients who are thought to have involved lymph nodes on pre-operative imaging (clinical N1 disease), surgery would ensure that all disease is removed. For these patients, removal of all disease in the chest (i.e., an R0 resection) confers significant prognostic benefit, as it has been shown that an R1 resection (i.e., with residual disease left behind in lymph nodes) confers a poorer prognosis that is comparable to disease left behind elsewhere in the chest (27). In contrast, SBRT should not be used to treat patients with clinical N1 disease, as SBRT only treats the primary lesion and not the surrounding lymph nodes (54).
In the case of patients who are thought to have negative lymph nodes based on pre-operative imaging, removal of the lymph nodes provides important information to ensure accurate staging of the lung cancer. A significant proportion of patients who present with negative nodal involvement on preoperative PET-CT scans will actually have positive nodal involvement upon subsequent histological examination of the lymph nodes post-surgery—approximately 7–14% of these patients will have occult N1 disease while 4–8% of them will have occult N2 disease (54-57). This results in the upstaging of their disease, and requires the prompt initiation of further adjuvant therapy to decrease their risk of recurrence, and to improve their overall survival (58). These upstaged patients would not be picked up using SBRT, as there is no opportunity for lymph node sampling with SBRT. Therefore, the subsequent care of these patients may be compromised without the proper staging of these patients.
Central lung lesions
Central tumors are those defined as being within 2 cm of the mediastinum and the proximal bronchial tree. Apart from the standard radiotoxicity seen in the SBRT treatment of peripheral lung tumors, central tumors present their own set of complications when treated by SBRT. An early study from Indiana University demonstrated that SBRT treatment of central tumors resulted in 11-fold more Grade 3–5 toxicity events as compared to SBRT treatment of peripheral tumors, and the investigators recommended against the use of SBRT in centrally located tumors (25). These results were subsequently confirmed by other studies from different groups, with reported complications including tracheoesophageal fistula, pneumonitis, stenosis/stricture, airway necrosis and fistula (16,59). Although some later studies have demonstrated that lower doses of radiation and the SBRT treatment of smaller central tumors might prove safer than higher doses of radiation and the SBRT treatment of larger central tumors, there is still no consensus on what would constitute an ideal SBRT regimen for the treatment of central tumors, and what the cut-off limit should be for tumor size (60,61).
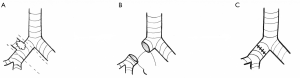
On the other hand, a conventional lobectomy can be used to safely remove lung tumors, regardless of whether they are peripheral or centrally located. Even in the event where a centrally located lung tumor directly involves the proximal airways or hilar vessels, thoracic surgeons are able to resect these lesions with good outcomes (62,63).While some of these lesions may require a pneumonectomy, often a sleeve resection can be performed to avoid the added morbidity from a pneumonectomy. In this case, part of the bronchus or pulmonary artery is resected above and below the tumor and rejoined, thus preserving pulmonary function (Figure 2). Outcomes of sleeve resections have compared favorably compared to pneumonectomies, and are comparable to conventional lobectomies (64-67). Thus, surgical resection should be considered the preferred treatment option for central tumors, as these tumors can be resected safely without the increased toxicity of SBRT (25,62,63).
Other lesions that cannot be treated effectively by SBRT
Apart from tumors located close to the mediastinum and the proximal bronchial tree, there are other lung tumors that cannot be effectively treated by SBRT. There have been reports that lower lobe NSCLC treated with SBRT have been associated with poorer outcomes (68), while no such difference in outcomes has been observed with surgical resection (69,70). Also, SBRT treatment of tumors close to the chest wall or diaphragm is technically challenging, but surgery with concomitant chest wall or diaphragm resection and reconstruction is frequently done with good outcomes (71-73). Similarly, patients with large tumors or multiple lesions are also easily treated with surgical resection, but are not good candidates for SBRT. An extended resection (pneumonectomy, bilobectomy, lobectomy with additional segmentectomy or wedge resection) may be performed to ensure all disease is removed at the time of surgery, provided the patient has adequate pulmonary function to tolerate the resection (74,75).
Overall survival and disease-free survival after surgery vs. SBRT
Given the relative advantages of both surgery and SBRT as discussed above, an area of recent interest is the comparison of surgery versus SBRT in treatment of early stage (typically defined as clinical stage I) NSCLC in medically operable patients, particularly those thought to be at higher risk for surgery (e.g., elderly or with many comorbidities). Given the encouraging results of the use of SBRT in early stage NSCLC in medically inoperable patients (10-14), many groups have published reports suggesting that the overall survival of medically operable patients with early stage NSCLC treated with SBRT have similar survival compared to patients who undergo surgical resection (43,44,47,76,77). However, many of these reports only studied the effects of SBRT on early stage NSCLC patients, and did not do a proper study comparing the two forms of treatment (44,47,76). These studies also often included patients who did not have a histological diagnosis of NSCLC, which would artificially improve their outcomes given that a proportion of these patients would actually have benign disease (42-44,47). This is compounded by the fact that these studies generally fail to clarify the imaging features of the primary lesion (i.e., whether it is solid versus a mixed or pure ground glass lesion), as ground glass lesions have significantly better outcomes because they are often an indolent form of NSCLC (78).
Of those studies that attempted to compare two forms of treatment, they were usually retrospective analyses, and some followed patients only for a short period of time post-treatment (77). In one study, it also included patients who underwent pneumonectomy in the surgical group, which could explain the very high 30-day mortality post-surgery in that study (43). Many of these studies also did not stage the patients properly before treatment, making it hard to directly compare survival data. [The SBRT group of these studies often did not do pre-treatment lymph node staging, so it is unclear whether these patients really had stage I disease. At the same time, in some studies, patients in the surgical groups who were upstaged after surgery due to the discovery of lymph node metastases after surgery were still analyzed as though they were part of the stage I cohort of patients (43)]. As such, the analyses of many of these papers claiming to show equivalency between surgery and SBRT in early NSCLC left much to be desired, and the conclusions the authors arrived at often would not hold up to a rigorous examination.
A major problem with attempting to compare surgery vs. SBRT directly is the lack of proper prospective randomized control trials to compare the two arms of treatment. Previous attempts at starting such a trial have failed due to problems with patient recruitment (46). In an attempt to salvage the data they have, the investigators in the STARS and ROSEL trial decided to combine their data and perform statistical analyses as though the two separate trials were one and the same. Unfortunately, even after doing so (and ignoring the problems with this method of treating two different trials with different patient populations as though it was one), their sample size was still much too small and the study was not powered to detect any difference between the two regimens. And as pointed out by many others (79-83), the authors of the “pooled analysis” have vastly overstated their perceived benefits of SBRT. The problems with their analysis include, among many others: (I) some of the patients were not required to undergo a biopsy to prove malignant disease before being enrolled in the trial; (II) patients were not adequately staged before the trial - some of the patients in the surgery arm were found during surgery to have later stages of NSCLC, and yet their results were included in the analysis; (III) a large proportion of patients did not reach the 3-year mark in their follow-up, even though the authors attempt to claim that SBRT is equivalent to surgery in terms of patient overall and disease-free survival 3-year post-treatment.
Given the lack of a properly conducted and adequately powered prospective randomized control trial, the best method we have available to compare surgery vs. SBRT are retrospective analyses using matched patient indicators, with a confirmed histological diagnosis of NSCLC. Patient indicators should be appropriately matched to reduce the confounding effects of any differences in patient characteristics between patients undergoing surgery vs. SBRT, such as patient age and existing comorbidities. If we look at the results of individual retrospective studies with these criteria, the results show that at 3–5 years post-treatment, patients who had surgical resection of their early stage lung cancer have better overall survival and recurrence-free survival than patients who underwent SBRT (84,85).
Similarly, if we look at studies analyzing large databases of patients treated with surgery or SBRT using the same criteria as above, we see that these studies consistently conclude that 3- and 5-year overall survival and cancer-specific survival favor patients who underwent surgery for their stage I NSCLC (53,86,87). A criticism of studies analyzing these large databases is that there might be a bias against SBRT in terms of overall survival, as patients who undergo SBRT are often in poorer health compared to patients who undergo surgery, so they may die from poor health rather than lung cancer per se. However, in the study conducted by Rosen and his colleagues, the authors selected patients from the National Cancer Database documented to be free of comorbidities, and still found a 5-year overall survival favoring surgery (59% vs. 29%). Even in their subgroup analysis of patients who underwent SBRT because they refused surgery matched to patients who underwent surgery, there was still a significant 5-year overall survival benefit favoring surgery (58% vs. 40%) (87).
Interestingly, if we look at database studies with histologically confirmed NSCLC comparing sublobar resections [in which a smaller section of the lung is removed rather than an entire lobe, and which is often thought to be inferior to lobectomy (1)] to SBRT, these studies still show that sublobar resections are superior to SBRT in terms of overall and lung cancer specific survival at 3 and 5 years post-treatment (35,53,88). This advantage is seen even in the group of patients who are older than 80 years of age, who are often considered to be very high risk for surgery (35).
Why would such a difference in survival exist between the two treatment arms for early stage NSCLC, given that SBRT has a much lower risk of immediate post-operative morbidity and mortality compared to surgery, and has been shown to have very high rates of local control? In a matched retrospective study comparing SBRT vs. surgery by Yu et al., it was shown that the toxicity associated with SBRT increases rapidly over time, such that by 24 months post-treatment, there was no significant difference in toxicity between the two treatment modalities. The same study also showed that while SBRT was associated with lower mortality initially, overall mortality was significantly higher for patients undergoing SBRT by 24-month post-therapy, as compared to patients who underwent surgical resection (89). Therefore, while SBRT appears to offer less serious complications compared to surgical resection in the immediate post-treatment period, this relative advantage wears off with time, and surgical resection offers better patient outcome in the long run. This is supported by results from a more recent study of the Veteran Affairs healthcare system database of early stage lung cancer patients, which demonstrated a higher mortality at 30 and 90 days after treatment, for surgically treated patients compared to patients treated with SBRT, but ultimately showed improved long-term overall and lung cancer specific survival for surgically treated patients compared to SBRT (86).
The future of thoracic surgery
Open thoracotomy used to be the standard treatment for early stage NSCLC, and it has served us well for decades. Long-term outcomes of thoracotomy-lobectomy specifically for stage I NSCLC have shown a 5-year overall survival rate of 62–81% (90-93). Studies providing the most recent information on thoracotomy-lobectomy outcomes report an overall morbidity of 32–37% and perioperative mortality of 1–2% (21,94,95). This represents an improvement from previously reported mortality of 2.9% for thoracotomy-lobectomy in the past (96). However, with further advances in thoracic surgery, we should be able to improve on these numbers.
The use of VATS allows us to perform lobectomies and other major lung resections with several keyhole incisions instead of a large thoracotomy incision. This decreases the amount of surgical trauma and pain for the patient, decreases the rate of complications, and also reduces recovery time (97-100). Various meta-analyses of VATS lobectomy compared to open lobectomy have also consistently shown improved 5-year survival rates with VATS lobectomy (97,101-103). For stage I NSCLC, VATS lobectomy provides a 5-year overall survival rate of 66–94% (90-93). Finally, VATS lobectomy complication rates range between 10–20% compared to thoracotomy-lobectomy’s 32–37%, with a similar perioperative mortality between the two modalities (95,97). Less surgical trauma also means that older, sicker patients which were previously classified as inoperable, may now be considered medically operable (100,104,105).
In the United States, where lobectomies may be performed by either thoracic surgeons or general surgeons, it has been demonstrated that thoracic surgeons perform more VATS lobectomies and complex thoracic operations compared to general surgeons (106), and lung cancer patient survival rates are higher in patients treated by thoracic surgeons compared to those treated by general surgeons (107,108). As VATS lobectomy adoption rates are still far from universal, it is hoped that by training more thoracic surgeons, and encouraging them to adopt the use of VATS rather than open lobectomy, the overall surgical outcome for NSCLC patients will improve with time (93,109).
Recent studies have also attempted to do a direct comparison between VATS lobectomy and SBRT in biopsy proven stage I NSCLC. Early results indicate that in propensity-matched patients who are studied retrospectively, patients who undergo VATS lobectomy had a better tumor control rate and better long-term overall and lung cancer-specific survival rates (84,85). The VATS lobectomy patients also had better recurrence-free survival, compared to the SBRT patients (84,85).
VATS lobectomy techniques have also been improving rapidly in recent years. Conventional multi-port VATS lobectomy is usually done with two to four port incisions, with three ports being the most common (110). With encouraging results from VATS-lobectomy, there is now growing interest worldwide in exploring whether further benefit can be derived by performing major lung resections through a single port incision (111,112). Early results have shown that uniportal VATS-lobectomy can be performed safely, and has some benefit in terms of postoperative pain control and length of stay as compared to multi-port VATS lobectomy (113-115).
Apart from improving the efficiency of VATS-lobectomy by decreasing the number of incisions used, thoracic surgeons are also investigating if parenchymal sparing or sublobar operations can further improve surgical outcomes in patients with very early stage NSCLC (<2 cm) (33,34,116). Although lobectomy was previously shown to be superior to sublobar operations based on a historical trial (1), there has been renewed attention in sublobar operations because they are better tolerated by patients and have lower rates of complications compared to lobectomy, as less lung tissue is resected (31,32,117). Two ongoing trials (CALGB140503 and JCOG0802) will provide insight into whether sublobar resections of small early stage tumours can be a non-inferior alternative compared to lobectomy (118,119).
Another area of interest within the thoracic surgical community is the concept of non-intubated thoracic surgery. A few select centers have shown that lobectomies, major lung resections and even complex airway operations can be safely performed without the need for general anesthesia (120-123). These operations are typically done under sedation with locoregional anesthesia, such as a thoracic epidural or intercostal nerve blockade (124,125). This holds great promise for decreasing morbidity from thoracic surgery, as the use of general anesthesia is associated with a higher risk of pneumonia and impaired cardiac function (126). However it remains to be seen whether these highly specialized techniques can be widely adopted by the general thoracic surgical community because of the inherent challenges in performing non-intubated thoracic surgery.
With all the advancements in surgical treatment of NSCLC, thoracic surgery for lung cancer patients should only get safer with time. As surgical techniques improve and complications decrease, more patients may become eligible for surgery, and SBRT may become reserved for the rare few stage I patients who are truly inoperable.
Conclusions
The standard treatment for early stage NSCLC is a lobectomy, which is safe for the vast majority of eligible patients. For patients who cannot tolerate a lobectomy, a sublobar resection is typically offered, although studies are still underway to define patient groups that would be best treated with a sublobar resection without compromising on long-term outcomes. SBRT is typically offered to patients who are not candidates for surgery, and there is now some interest in expanding the use of SBRT to medically operable patients as well, in view of the fact that SBRT appears to result in lower rates of complications and less downtime for patients, as compared to an open operation.
However, there are no good prospective trials comparing the outcomes of lobectomy vs. SBRT in stage I NSCLC patients, and unfortunately it appears unlikely that a credible trial will be conducted any time soon, due to poor patient recruitment in previous attempts at these trials. Many existing studies claiming that SBRT is comparable to surgery in lung cancer should be viewed with caution due to their inherent flaws, such as a lack of a histological diagnosis of the primary tumor or histological confirmation of nodal involvement. Other matched retrospective analyses and large database studies with a confirmed histological diagnosis of NSCLC tend to suggest that surgery remains the superior option, with better disease control and patient survival. While retrospective studies may miss certain confounding factors, these studies are balanced by the large numbers of propensity-matched patients included in the analyses. Clearly, more high-quality studies comparing surgery and SBRT need to be carried out, but at the moment, any evidence claiming equivalency between the two procedures is weak.
A central difference in comparing surgery vs. SBRT, is that surgical resection for lung cancer is a diagnostic, staging and therapeutic procedure all at the same time. Surgical resection confirms the histopathological diagnosis and provides additional staging information while removing the lung tumor. On the other hand, SBRT is solely a therapeutic procedure relying mainly on radiological imaging (and its attendant limitations) to convey information that will be incomplete without surgical resection. Surgery offers an advantage over SBRT in terms of the ability to remove the primary tumor and the surrounding lymph nodes during the operation, on which we can conduct histopathological studies that will provide us with information regarding the adequacy of treatment, allows for more accurate staging of the disease, and also alert us to the presence of microscopic dissemination of the tumor cells. The histopathological analysis can then provide a guide as to whether the patient requires further treatment and follow-up.
Furthermore, with advances in techniques and instrumentation, surgical outcomes are expected to improve and the pool of medically operable patients should likewise expand. Minimally invasive VATS lobectomy is now commonly performed as an alternative to thoracotomy-lobectomy, resulting in decreased rates of complications and improved patient survival. Recent studies involving matched analyses of VATS lobectomy vs. SBRT have also shown VATS lobectomy to be the superior therapy, and with further refinement in surgical techniques (e.g., uniportal VATS, parenchymal sparing surgery and non-intubated thoracic surgery), it can be expected that lung cancer surgery will only get safer with time. Therefore, surgical resection (preferably via VATS) should remain the treatment of choice for lung cancer patients who are eligible for surgery, until conclusively proven otherwise.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Kam-Weng Fong, Kevin Lee Min Chua) for the series “Radiotherapy in Lung Cancer” published in Journal of Xiangya Medicine. The article has undergone external peer review.
Conflicts of Interest: The author has completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jxym.2018.06.01). The series “Radiotherapy in Lung Cancer” was commissioned by the editorial office without any funding or sponsorship. BHO reports non-financial support from Johnson & Johnson, non-financial support from Medtronic, non-financial support from Broncus, outside the submitted work. The author has no other conflicts of interest to declare.
Ethical Statement: The author is accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ginsberg RJ, Rubinstein LV. Randomized trial of lobectomy versus limited resection for T1 N0 non-small cell lung cancer. Lung Cancer Study Group. Ann Thorac Surg 1995;60:615-22; discussion 22-3. [Crossref] [PubMed]
- Lim E, Baldwin D, Beckles M, et al. Guidelines on the radical management of patients with lung cancer. Thorax 2010;65:iii1-27. [Crossref] [PubMed]
- Brunelli A, Kim AW, Berger KI, et al. Physiologic evaluation of the patient with lung cancer being considered for resectional surgery: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:e166S-e90S.
- Goldstraw P, Chansky K, Crowley J, et al. The IASLC Lung Cancer Staging Project: Proposals for Revision of the TNM Stage Groupings in the Forthcoming (Eighth) Edition of the TNM Classification for Lung Cancer. J Thorac Oncol 2016;11:39-51. [Crossref] [PubMed]
- Lasak JM, Gorecki JP. The history of stereotactic radiosurgery and radiotherapy. Otolaryngol Clin North Am 2009;42:593-9. [Crossref] [PubMed]
- Timmerman RD, Herman J, Cho LC. Emergence of stereotactic body radiation therapy and its impact on current and future clinical practice. J Clin Oncol 2014;32:2847-54. [Crossref] [PubMed]
- Chua KLM, Sin I, Fong KW, et al. Stereotactic body radiotherapy for early stage lung cancer-historical developments and future strategies. Chin Clin Oncol 2017;6:S20. [Crossref] [PubMed]
- Pennathur A, Luketich JD, Burton S, et al. Stereotactic radiosurgery for the treatment of lung neoplasm: initial experience. Ann Thorac Surg 2007;83:1820-4; discussion 4-5.
- Pennathur A, Luketich JD, Heron DE, et al. Stereotactic radiosurgery for the treatment of stage I non-small cell lung cancer in high-risk patients. J Thorac Cardiovasc Surg 2009;137:597-604. [Crossref] [PubMed]
- Nanda RH, Liu Y, Gillespie TW, et al. Stereotactic body radiation therapy versus no treatment for early stage non-small cell lung cancer in medically inoperable elderly patients: A National Cancer Data Base analysis. Cancer 2015;121:4222-30. [Crossref] [PubMed]
- Timmerman R, Paulus R, Galvin J, et al. Stereotactic body radiation therapy for inoperable early stage lung cancer. JAMA 2010;303:1070-6. [Crossref] [PubMed]
- Jeppesen SS, Schytte T, Jensen HR, et al. Stereotactic body radiation therapy versus conventional radiation therapy in patients with early stage non-small cell lung cancer: an updated retrospective study on local failure and survival rates. Acta Oncol 2013;52:1552-8. [Crossref] [PubMed]
- Abreu CE, Ferreira PP, de Moraes FY, et al. Stereotactic body radiotherapy in lung cancer: an update. J Bras Pneumol 2015;41:376-87. [Crossref] [PubMed]
- Nyman J, Hallqvist A, Lund J, et al. SPACE - A randomized study of SBRT vs. conventional fractionated radiotherapy in medically inoperable stage I NSCLC. Radiother Oncol 2016;121:1-8. [Crossref] [PubMed]
- Ricardi U, Filippi AR, Guarneri A, et al. Stereotactic body radiation therapy for early stage non-small cell lung cancer: results of a prospective trial. Lung Cancer 2010;68:72-7. [Crossref] [PubMed]
- Kang KH, Okoye CC, Patel RB, et al. Complications from Stereotactic Body Radiotherapy for Lung Cancer. Cancers (Basel) 2015;7:981-1004. [Crossref] [PubMed]
- Cerfolio RJ, Bass CS, Pask AH, et al. Predictors and treatment of persistent air leaks. Ann Thorac Surg 2002;73:1727-30; discussion 30-1.
- Varela G, Jiménez MF, Novoa N, et al. Estimating hospital costs attributable to prolonged air leak in pulmonary lobectomy. Eur J Cardiothorac Surg 2005;27:329-33. [Crossref] [PubMed]
- Lee L, Hanley SC, Robineau C, et al. Estimating the risk of prolonged air leak after pulmonary resection using a simple scoring system. J Am Coll Surg 2011;212:1027-32. [Crossref] [PubMed]
- Elsayed H, McShane J, Shackcloth M. Air leaks following pulmonary resection for lung cancer: is it a patient or surgeon related problem? Ann R Coll Surg Engl 2012;94:422-7. [Crossref] [PubMed]
- Allen MS, Darling GE, Pechet TT, et al. Morbidity and mortality of major pulmonary resections in patients with early-stage lung cancer: initial results of the randomized, prospective ACOSOG Z0030 trial. Ann Thorac Surg 2006;81:1013-9; discussion 9-20. [Crossref] [PubMed]
- Ziarnik E, Grogan EL. Postlobectomy Early Complications. Thorac Surg Clin 2015;25:355-64. [Crossref] [PubMed]
- Chi A, Liao Z, Nguyen NP, et al. Systemic review of the patterns of failure following stereotactic body radiation therapy in early-stage non-small-cell lung cancer: clinical implications. Radiother Oncol 2010;94:1-11. [Crossref] [PubMed]
- Baumann P, Nyman J, Hoyer M, et al. Stereotactic body radiotherapy for medically inoperable patients with stage I non-small cell lung cancer - a first report of toxicity related to COPD/CVD in a non-randomized prospective phase II study. Radiother Oncol 2008;88:359-67. [Crossref] [PubMed]
- Timmerman R, McGarry R, Yiannoutsos C, et al. Excessive toxicity when treating central tumors in a phase II study of stereotactic body radiation therapy for medically inoperable early-stage lung cancer. J Clin Oncol 2006;24:4833-9. [Crossref] [PubMed]
- Hofmann HS, Taege C, Lautenschläger C, et al. Microscopic (R1) and macroscopic (R2) residual disease in patients with resected non-small cell lung cancer. Eur J Cardiothorac Surg 2002;21:606-10. [Crossref] [PubMed]
- Riquet M, Achour K, Foucault C, et al. Microscopic residual disease after resection for lung cancer: a multifaceted but poor factor of prognosis. Ann Thorac Surg 2010;89:870-5. [Crossref] [PubMed]
- Hancock JG, Rosen JE, Antonicelli A, et al. Impact of adjuvant treatment for microscopic residual disease after non-small cell lung cancer surgery. Ann Thorac Surg 2015;99:406-13. [Crossref] [PubMed]
- Bradley J. Radiographic response and clinical toxicity following SBRT for stage I lung cancer. J Thorac Oncol 2007;2:S118-24. [Crossref] [PubMed]
- Dahele M, Palma D, Lagerwaard F, et al. Radiological changes after stereotactic radiotherapy for stage I lung cancer. J Thorac Oncol 2011;6:1221-8. [Crossref] [PubMed]
- Gulack BC, Yang CJ, Speicher PJ, et al. A Risk Score to Assist Selecting Lobectomy Versus Sublobar Resection for Early Stage Non-Small Cell Lung Cancer. Ann Thorac Surg 2016;102:1814-20. [Crossref] [PubMed]
- Kilic A, Schuchert MJ, Pettiford BL, et al. Anatomic segmentectomy for stage I non-small cell lung cancer in the elderly. Ann Thorac Surg 2009;87:1662-6; discussion 7-8.
- Altorki NK, Yip R, Hanaoka T, et al. Sublobar resection is equivalent to lobectomy for clinical stage 1A lung cancer in solid nodules. J Thorac Cardiovasc Surg 2014;147:754-62; Discussion 62-4. [Crossref] [PubMed]
- Cao C, Gupta S, Chandrakumar D, et al. Meta-analysis of intentional sublobar resections versus lobectomy for early stage non-small cell lung cancer. Ann Cardiothorac Surg 2014;3:134-41. [PubMed]
- Yerokun BA, Yang CJ, Gulack BC, et al. A national analysis of wedge resection versus stereotactic body radiation therapy for stage IA non-small cell lung cancer. J Thorac Cardiovasc Surg 2017;154:675-86.e4. [Crossref] [PubMed]
- Stiles BM, Kamel MK, Nasar A, et al. The importance of lymph node dissection accompanying wedge resection for clinical stage IA lung cancer. Eur J Cardiothorac Surg 2017;51:511-7. [PubMed]
- Carillo GA, Vázquez JE, Villar AF. Prevalence of benign pulmonary lesions excised for suspicion of malignancy: could it reflect a quality management index of indeterminate lung lesions? Korean J Thorac Cardiovasc Surg 2014;47:458-64. [Crossref] [PubMed]
- Maiga AW, Deppen SA, Pinkerman R, et al. A Successful Institutional Strategy to Increase the Number of Therapeutic Operations Among Patients With Lung Lesions. JAMA Surg 2016;151:193-4. [Crossref] [PubMed]
- Kohman LJ, Gu L, Altorki N, et al. Biopsy first: Lessons learned from Cancer and Leukemia Group B (CALGB) 140503. J Thorac Cardiovasc Surg 2017;153:1592-7. [Crossref] [PubMed]
- Wahidi MM, Govert JA, Goudar RK, et al. Evidence for the treatment of patients with pulmonary nodules: when is it lung cancer?: ACCP evidence-based clinical practice guidelines (2nd edition). Chest 2007;132:94S-107S.
- Sim YT, Goh YG, Dempsey MF, et al. PET-CT evaluation of solitary pulmonary nodules: correlation with maximum standardized uptake value and pathology. Lung 2013;191:625-32. [Crossref] [PubMed]
- Shaikh T, Churilla TM, Murphy CT, et al. Absence of Pathological Proof of Cancer Associated with Improved Outcomes in Early-Stage Lung Cancer. J Thorac Oncol 2016;11:1112-20. [Crossref] [PubMed]
- Palma D, Visser O, Lagerwaard FJ, et al. Treatment of stage I NSCLC in elderly patients: a population-based matched-pair comparison of stereotactic radiotherapy versus surgery. Radiother Oncol 2011;101:240-4. [Crossref] [PubMed]
- Lagerwaard FJ, Verstegen NE, Haasbeek CJ, et al. Outcomes of stereotactic ablative radiotherapy in patients with potentially operable stage I non-small cell lung cancer. Int J Radiat Oncol Biol Phys 2012;83:348-53. [Crossref] [PubMed]
- Kastelijn EA, El Sharouni SY, Hofman FN, et al. Clinical Outcomes in Early-stage NSCLC Treated with Stereotactic Body Radiotherapy Versus Surgical Resection. Anticancer Res 2015;35:5607-14. [PubMed]
- Chang JY, Senan S, Paul MA, et al. Stereotactic ablative radiotherapy versus lobectomy for operable stage I non-small-cell lung cancer: a pooled analysis of two randomised trials. Lancet Oncol 2015;16:630-7. [Crossref] [PubMed]
- Eriguchi T, Takeda A, Sanuki N, et al. Stereotactic body radiotherapy for operable early-stage non-small cell lung cancer. Lung Cancer 2017;109:62-7. [Crossref] [PubMed]
- Crabtree TD, Puri V, Robinson C, et al. Analysis of first recurrence and survival in patients with stage I non-small cell lung cancer treated with surgical resection or stereotactic radiation therapy. J Thorac Cardiovasc Surg 2014;147:1183-91; discussion 91-2. [Crossref] [PubMed]
- Port JL, Parashar B, Osakwe N, et al. A propensity-matched analysis of wedge resection and stereotactic body radiotherapy for early stage lung cancer. Ann Thorac Surg 2014;98:1152-9. [Crossref] [PubMed]
- Morgensztern D, Du L, Waqar SN, et al. Adjuvant Chemotherapy for Patients with T2N0M0 NSCLC. J Thorac Oncol 2016;11:1729-35. [Crossref] [PubMed]
- Higgins KA, Chino JP, Ready N, et al. Lymphovascular invasion in non-small-cell lung cancer: implications for staging and adjuvant therapy. J Thorac Oncol 2012;7:1141-7. [Crossref] [PubMed]
- Qian F, Yang W, Wang R, et al. Prognostic significance and adjuvant chemotherapy survival benefits of a solid or micropapillary pattern in patients with resected stage IB lung adenocarcinoma. J Thorac Cardiovasc Surg 2018;155:1227-35.e2. [Crossref] [PubMed]
- Puri V, Crabtree TD, Bell JM, et al. Treatment Outcomes in Stage I Lung Cancer: A Comparison of Surgery and Stereotactic Body Radiation Therapy. J Thorac Oncol 2015;10:1776-84. [Crossref] [PubMed]
- Akthar AS, Ferguson MK, Koshy M, et al. Limitations of PET/CT in the Detection of Occult N1 Metastasis in Clinical Stage I(T1-2aN0) Non-Small Cell Lung Cancer for Staging Prior to Stereotactic Body Radiotherapy. Technol Cancer Res Treat 2017;16:15-21. [Crossref] [PubMed]
- Park HK, Jeon K, Koh WJ, et al. Occult nodal metastasis in patients with non-small cell lung cancer at clinical stage IA by PET/CT. Respirology 2010;15:1179-84. [Crossref] [PubMed]
- Ong P, Grosu H, Eapen GA, et al. Endobronchial ultrasound-guided transbronchial needle aspiration for systematic nodal staging of lung cancer in patients with N0 disease by computed tomography and integrated positron emission tomography-computed tomography. Ann Am Thorac Soc 2015;12:415-9. [Crossref] [PubMed]
- Lin JT, Yang XN, Zhong WZ, et al. Association of maximum standardized uptake value with occult mediastinal lymph node metastases in cN0 non-small cell lung cancer. Eur J Cardiothorac Surg 2016;50:914-9. [Crossref] [PubMed]
- Winton T, Livingston R, Johnson D, et al. Vinorelbine plus cisplatin vs. observation in resected non-small-cell lung cancer. N Engl J Med 2005;352:2589-97. [Crossref] [PubMed]
- Song SY, Choi W, Shin SS, et al. Fractionated stereotactic body radiation therapy for medically inoperable stage I lung cancer adjacent to central large bronchus. Lung Cancer 2009;66:89-93. [Crossref] [PubMed]
- Modh A, Rimner A, Williams E, et al. Local control and toxicity in a large cohort of central lung tumors treated with stereotactic body radiation therapy. Int J Radiat Oncol Biol Phys 2014;90:1168-76. [Crossref] [PubMed]
- Davis JN, Medbery C, Sharma S, et al. Stereotactic body radiotherapy for centrally located early-stage non-small cell lung cancer or lung metastases from the RSSearch(®) patient registry. Radiat Oncol 2015;10:113. [Crossref] [PubMed]
- Tronc F, Grégoire J, Rouleau J, et al. Long-term results of sleeve lobectomy for lung cancer. Eur J Cardiothorac Surg 2000;17:550-6. [Crossref] [PubMed]
- Merritt RE, Mathisen DJ, Wain JC, et al. Long-term results of sleeve lobectomy in the management of non-small cell lung carcinoma and low-grade neoplasms. Ann Thorac Surg 2009;88:1574-81; discussion 81-2. [Crossref] [PubMed]
- Shi W, Zhang W, Sun H, et al. Sleeve lobectomy versus pneumonectomy for non-small cell lung cancer: a meta-analysis. World J Surg Oncol 2012;10:265. [Crossref] [PubMed]
- Park JS, Yang HC, Kim HK, et al. Sleeve lobectomy as an alternative procedure to pneumonectomy for non-small cell lung cancer. J Thorac Oncol 2010;5:517-20. [Crossref] [PubMed]
- Ma Z, Dong A, Fan J, et al. Does sleeve lobectomy concomitant with or without pulmonary artery reconstruction (double sleeve) have favorable results for non-small cell lung cancer compared with pneumonectomy? A meta-analysis. Eur J Cardiothorac Surg 2007;32:20-8. [Crossref] [PubMed]
- Kojima F, Yamamoto K, Matsuoka K, et al. Factors affecting survival after lobectomy with pulmonary artery resection for primary lung cancer. Eur J Cardiothorac Surg 2011;40:e13-20. [Crossref] [PubMed]
- Shaverdian N, Veruttipong D, Wang J, et al. Location Matters: Stage I Non-Small-cell Carcinomas of the Lower Lobes Treated With Stereotactic Body Radiation Therapy Are Associated With Poor Outcomes. Clin Lung Cancer 2017;18:e137-e42. [Crossref] [PubMed]
- Whitson BA, Groth SS, Andrade RS, et al. T1/T2 non-small-cell lung cancer treated by lobectomy: does tumor anatomic location matter? J Surg Res 2012;177:185-90. [Crossref] [PubMed]
- Puri V, Garg N, Engelhardt EE, et al. Tumor location is not an independent prognostic factor in early stage non-small cell lung cancer. Ann Thorac Surg 2010;89:1053-9. [Crossref] [PubMed]
- Watanabe Y, Shimizu J, Oda M, et al. Results of surgical treatment in patients with stage IIIA non-small-cell lung cancer. Thorac Cardiovasc Surg 1991;39:44-9. [Crossref] [PubMed]
- Doddoli C, D'Journo B, Le Pimpec-Barthes F, et al. Lung cancer invading the chest wall: a plea for en-bloc resection but the need for new treatment strategies. Ann Thorac Surg 2005;80:2032-40. [Crossref] [PubMed]
- Galetta D, Borri A, Casiraghi M, et al. Outcome and prognostic factors of resected non-small-cell lung cancer invading the diaphragm. Interact Cardiovasc Thorac Surg 2014;19:632-6; discussion 6. [Crossref] [PubMed]
- Izbicki JR, Knoefel WT, Passlick B, et al. Risk analysis and long-term survival in patients undergoing extended resection of locally advanced lung cancer. J Thorac Cardiovasc Surg 1995;110:386-95. [Crossref] [PubMed]
- Thomas PA, Falcoz PE, Bernard A, et al. Bilobectomy for lung cancer: contemporary national early morbidity and mortality outcomes. Eur J Cardiothorac Surg 2016;49:e38-43. discussion e. [Crossref] [PubMed]
- Onishi H, Shirato H, Nagata Y, et al. Stereotactic body radiotherapy (SBRT) for operable stage I non-small-cell lung cancer: can SBRT be comparable to surgery? Int J Radiat Oncol Biol Phys 2011;81:1352-8. [Crossref] [PubMed]
- Varlotto J, Fakiris A, Flickinger J, et al. Matched-pair and propensity score comparisons of outcomes of patients with clinical stage I non-small cell lung cancer treated with resection or stereotactic radiosurgery. Cancer 2013;119:2683-91. [Crossref] [PubMed]
- Sagawa M, Oizumi H, Suzuki H, et al. A prospective 5-year follow-up study after limited resection for lung cancer with ground-glass opacity. Eur J Cardiothorac Surg 2018;53:849-56. [Crossref] [PubMed]
- Opitz I, Rocco G, Brunelli A, et al. Surgery versus SABR for resectable non-small-cell lung cancer. Lancet Oncol 2015;16:e372-3. [Crossref] [PubMed]
- Hamaji M, Groth SS, Sugarbaker DJ, et al. Surgery versus SABR for resectable non-small-cell lung cancer. Lancet Oncol 2015;16:e372. [Crossref] [PubMed]
- Zhang L, Tian J, Wang C. Surgery versus SABR for resectable non-small-cell lung cancer. Lancet Oncol 2015;16:e371-2. [Crossref] [PubMed]
- Cao C, D'Amico T, Demmy T, et al. Surgery versus SABR for resectable non-small-cell lung cancer. Lancet Oncol 2015;16:e370-1. [Crossref] [PubMed]
- Meyers BF, Puri V, Broderick SR, et al. Lobectomy versus stereotactic body radiotherapy for stage I non-small cell lung cancer: Post hoc analysis dressed up as level-1 evidence? J Thorac Cardiovasc Surg 2015;150:468-71. [Crossref] [PubMed]
- Hamaji M, Chen F, Matsuo Y, et al. Video-assisted thoracoscopic lobectomy versus stereotactic radiotherapy for stage I lung cancer. Ann Thorac Surg 2015;99:1122-9. [Crossref] [PubMed]
- Cornwell LD, Echeverria AE, Samuelian J, et al. Video-assisted thoracoscopic lobectomy is associated with greater recurrence-free survival than stereotactic body radiotherapy for clinical stage I lung cancer. J Thorac Cardiovasc Surg 2018;155:395-402. [Crossref] [PubMed]
- Bryant AK, Mundt RC, Sandhu AP, et al. Stereotactic Body Radiation Therapy Versus Surgery for Early Lung Cancer Among US Veterans. Ann Thorac Surg 2018;105:425-31. [Crossref] [PubMed]
- Rosen JE, Salazar MC, Wang Z, et al. Lobectomy versus stereotactic body radiotherapy in healthy patients with stage I lung cancer. J Thorac Cardiovasc Surg 2016;152:44-54.e9. [Crossref] [PubMed]
- Ezer N, Veluswamy RR, Mhango G, et al. Outcomes after Stereotactic Body Radiotherapy versus Limited Resection in Older Patients with Early-Stage Lung Cancer. J Thorac Oncol 2015;10:1201-6. [Crossref] [PubMed]
- Yu JB, Soulos PR, Cramer LD, et al. Comparative effectiveness of surgery and radiosurgery for stage I non-small cell lung cancer. Cancer 2015;121:2341-9. [Crossref] [PubMed]
- Higuchi M, Yaginuma H, Yonechi A, et al. Long-term outcomes after video-assisted thoracic surgery (VATS) lobectomy versus lobectomy via open thoracotomy for clinical stage IA non-small cell lung cancer. J Cardiothorac Surg 2014;9:88. [Crossref] [PubMed]
- Lee PC, Nasar A, Port JL, et al. Long-term survival after lobectomy for non-small cell lung cancer by video-assisted thoracic surgery versus thoracotomy. Ann Thorac Surg 2013;96:951-60; discussion 60-1. [Crossref] [PubMed]
- Kuritzky AM, Aswad BI, Jones RN, et al. Lobectomy by Video-Assisted Thoracic Surgery vs. Muscle-Sparing Thoracotomy for Stage I Lung Cancer: A Critical Evaluation of Short- and Long-Term Outcomes. J Am Coll Surg 2015;220:1044-53. [Crossref] [PubMed]
- Yang CJ, Kumar A, Klapper JA, et al. A National Analysis of Long-term Survival Following Thoracoscopic Versus Open Lobectomy for Stage I Non-small-cell Lung Cancer. Ann Surg 2017; [Epub ahead of print]. [Crossref] [PubMed]
- Boffa DJ, Allen MS, Grab JD, et al. Data from The Society of Thoracic Surgeons General Thoracic Surgery database: the surgical management of primary lung tumors. J Thorac Cardiovasc Surg 2008;135:247-54. [Crossref] [PubMed]
- Rueth NM, Andrade RS. Is VATS lobectomy better: perioperatively, biologically and oncologically? Ann Thorac Surg 2010;89:S2107-11. [Crossref] [PubMed]
- Ginsberg RJ, Hill LD, Eagan RT, et al. Modern thirty-day operative mortality for surgical resections in lung cancer. J Thorac Cardiovasc Surg 1983;86:654-8. [PubMed]
- Whitson BA, Groth SS, Duval SJ, et al. Surgery for early-stage non-small cell lung cancer: a systematic review of the video-assisted thoracoscopic surgery versus thoracotomy approaches to lobectomy. Ann Thorac Surg 2008;86:2008-16; discussion 16-8.
- Cao C, Manganas C, Ang SC, et al. A systematic review and meta-analysis on pulmonary resections by robotic video-assisted thoracic surgery. Ann Cardiothorac Surg 2012;1:3-10. [PubMed]
- Paul S, Altorki NK, Sheng S, et al. Thoracoscopic lobectomy is associated with lower morbidity than open lobectomy: a propensity-matched analysis from the STS database. J Thorac Cardiovasc Surg 2010;139:366-78. [Crossref] [PubMed]
- Wang Y. Video-assisted thoracoscopic surgery for non-small-cell lung cancer is beneficial to elderly patients. Int J Clin Exp Med 2015;8:13604-9. [PubMed]
- Yan TD, Black D, Bannon PG, et al. Systematic review and meta-analysis of randomized and nonrandomized trials on safety and efficacy of video-assisted thoracic surgery lobectomy for early-stage non-small-cell lung cancer. J Clin Oncol 2009;27:2553-62. [Crossref] [PubMed]
- Chen FF, Zhang D, Wang YL, et al. Video-assisted thoracoscopic surgery lobectomy versus open lobectomy in patients with clinical stage I non-small cell lung cancer: a meta-analysis. Eur J Surg Oncol 2013;39:957-63. [Crossref] [PubMed]
- Taioli E, Lee DS, Lesser M, et al. Long-term survival in video-assisted thoracoscopic lobectomy vs. open lobectomy in lung-cancer patients: a meta-analysis. Eur J Cardiothorac Surg 2013;44:591-7. [Crossref] [PubMed]
- Oparka J, Yan TD, Ryan E, et al. Does video-assisted thoracic surgery provide a safe alternative to conventional techniques in patients with limited pulmonary function who are otherwise suitable for lung resection? Interact Cardiovasc Thorac Surg 2013;17:159-62. [Crossref] [PubMed]
- Donahoe LL, de Valence M, Atenafu EG, et al. High Risk for Thoracotomy but not Thoracoscopic Lobectomy. Ann Thorac Surg 2017;103:1730-5. [Crossref] [PubMed]
- Cooke DT, Wisner DH. Who performs complex noncardiac thoracic surgery in United States academic medical centers? Ann Thorac Surg 2012;94:1060-4. [Crossref] [PubMed]
- Farjah F, Flum DR, Varghese TK, et al. Surgeon specialty and long-term survival after pulmonary resection for lung cancer. Ann Thorac Surg 2009;87:995-1004; discussion 5-6. [Crossref] [PubMed]
- Ellis MC, Diggs BS, Vetto JT, et al. Intraoperative oncologic staging and outcomes for lung cancer resection vary by surgeon specialty. Ann Thorac Surg 2011;92:1958-63; discussion 63-4.
- Blasberg JD, Seder CW, Leverson G, et al. Video-Assisted Thoracoscopic Lobectomy for Lung Cancer: Current Practice Patterns and Predictors of Adoption. Ann Thorac Surg 2016;102:1854-62. [Crossref] [PubMed]
- Gonzalez-Rivas D. VATS lobectomy: surgical evolution from conventional VATS to uniportal approach. ScientificWorldJournal 2012;2012:780842. [Crossref] [PubMed]
- Drevet G, Ugalde Figueroa P. Uniportal video-assisted thoracoscopic surgery: safety, efficacy and learning curve during the first 250 cases in Quebec, Canada. Ann Cardiothorac Surg 2016;5:100-6. [Crossref] [PubMed]
- Reinersman JM, Passera E, Rocco G. Overview of uniportal video-assisted thoracic surgery (VATS): past and present. Ann Cardiothorac Surg 2016;5:112-7. [Crossref] [PubMed]
- Harris CG, James RS, Tian DH, et al. Systematic review and meta-analysis of uniportal versus multiportal video-assisted thoracoscopic lobectomy for lung cancer. Ann Cardiothorac Surg 2016;5:76-84. [Crossref] [PubMed]
- Wang L, Liu D, Lu J, et al. The feasibility and advantage of uniportal video-assisted thoracoscopic surgery (VATS) in pulmonary lobectomy. BMC Cancer 2017;17:75. [Crossref] [PubMed]
- Abouarab AA, Rahouma M, Kamel M, et al. Single Versus Multi-Incisional Video-Assisted Thoracic Surgery: A Systematic Review and Meta-analysis. J Laparoendosc Adv Surg Tech A 2018;28:174-85. [Crossref] [PubMed]
- Schuchert MJ, Kilic A, Pennathur A, et al. Oncologic outcomes after surgical resection of subcentimeter non-small cell lung cancer. Ann Thorac Surg 2011;91:1681-7; discussion 7-8.
- Razi SS, John MM, Sainathan S, et al. Sublobar resection is equivalent to lobectomy for T1a non-small cell lung cancer in the elderly: a Surveillance, Epidemiology, and End Results database analysis. J Surg Res 2016;200:683-9. [Crossref] [PubMed]
- Wolf AS, Richards WG, Jaklitsch MT, et al. Lobectomy versus sublobar resection for small (2 cm or less) non-small cell lung cancers. Ann Thorac Surg 2011;92:1819-23; discussion 24-5.
- Nakamura K, Saji H, Nakajima R, et al. A phase III randomized trial of lobectomy versus limited resection for small-sized peripheral non-small cell lung cancer (JCOG0802/WJOG4607L). Jpn J Clin Oncol 2010;40:271-4. [Crossref] [PubMed]
- Wang ML, Galvez C, Chen JS, et al. Non-intubated single-incision video-assisted thoracic surgery: a two-center cohort of 188 patients. J Thorac Dis 2017;9:2587-98. [Crossref] [PubMed]
- Liu J, Cui F, Pompeo E, et al. The impact of non-intubated versus intubated anaesthesia on early outcomes of video-assisted thoracoscopic anatomical resection in non-small-cell lung cancer: a propensity score matching analysis. Eur J Cardiothorac Surg 2016;50:920-5. [Crossref] [PubMed]
- Peng G, Cui F, Ang KL, et al. Non-intubated combined with video-assisted thoracoscopic in carinal reconstruction. J Thorac Dis 2016;8:586-93. [Crossref] [PubMed]
- Mineo TC, Tamburrini A, Perroni G, et al. 1000 cases of tubeless video-assisted thoracic surgery at the Rome Tor Vergata University. Future Oncol 2016;12:13-8. [Crossref] [PubMed]
- Zhao ZR, Lau RW, Ng CS. Anaesthesiology for uniportal VATS: double lumen, single lumen and tubeless. J Vis Surg 2017;3:108. [Crossref] [PubMed]
- Deng HY, Zhu ZJ, Wang YC, et al. Non-intubated video-assisted thoracoscopic surgery under loco-regional anaesthesia for thoracic surgery: a meta-analysis. Interact Cardiovasc Thorac Surg 2016;23:31-40. [Crossref] [PubMed]
- Gonzalez-Rivas D, Bonome C, Fieira E, et al. Non-intubated video-assisted thoracoscopic lung resections: the future of thoracic surgery? Eur J Cardiothorac Surg 2016;49:721-31. [Crossref] [PubMed]
Cite this article as: Ong BH. Surgery versus stereotactic body radiotherapy in medically operable non-small cell lung cancer. J Xiangya Med 2018;3:26.