
Distally based anterolateral thigh flap pedicled on the oblique branch of the lateral circumflex femoral artery
Introduction
Since Zhang (1) first described the use of distally based anterolateral thigh (dALT) flaps, pertinent data regarding anatomical basis, arterial supply and venous drainage, and clinical applications have been well-documented. The lateral circumflex femoral artery (LCFA) typically divides into ascending, transverse, and descending branches shortly after it originates from the profunda femoral artery. The descending branch first travels between the rectus and vastus intermedius muscles and then along the medial border of the vastus lateralis muscle. The descending branch sends various cutaneous and muscular branches to nourish the skin and muscles of the anterolateral and anteromedial thigh regions. The terminal branches of the descending branch enter into the vastus lateralis muscle at different levels above the knee and anastomose with the articular geniculate network, particularly the superior lateral genicular artery (2). The anterolateral thigh flap (ALT) was traditionally based on the perforator or cutaneous branches from the LCFA descending branch. A dALT flap is supplied by the reverse blood flow from the distal portion of the LCFA descending branch when the proximal portion of the descending branch is ligated. The principal drawbacks of the dALT flap include anatomic variations, pedicle length limitations, and occasional venous congestion.
Pan et al. (2004) (3) carried out a detailed anatomical study of the dALT flap and found that the perforators on which a dALT flap is based may arise from the descending and transverse branches of the LCFA. In 2009, Wong et al. (4) first defined a previously unnamed branch of the ALT region as the LCFA oblique branch and demonstrated the clinical significance of this branch in raising an ALT flap. To the best of our knowledge, a dALT flap based on the LCFA oblique branch has not been reported in the literature. This article presents our preliminary observations on the usage of the LCFA oblique branch in dALT flaps, and our experiences in reconstructing lower extremity defects using a dALT flap based on the LCFA oblique branch. Our study also highlights the clinical significance and potential usage of the LCFA oblique branch in reconstructive strategies.
Methods
All procedures were approved by the Institutional Review Board of the Chinese Academy of Medical Science and Peking Union Medical College prior to the execution of the study. Patients and/or direct family members gave written consent to the procedure. The present study strictly abides by the Declaration of Helsinki.
Between 2008 and 2016, 18 consecutive dALT flaps in 18 patients were transferred for reconstruction of lower extremity defects around the knee or the proximal leg. Among these patients, seven flaps were based on the perforators of the oblique branch of the LCFA. Of these patients four were male and three were female. Age ranged from 3 to 58 years (mean, 34 years). The patients had soft tissue defects that were caused by resection of post-burn scars (four patients) or malignant tumor (three patients). Patient demographics are presented in Table 1.
Table 1
| No. | Sex/age (y) | Side | Cause of defect | Defect location | Perforator pattern | Flap size (cm) | Pedicle length (cm) | Length of the AP line | Pivot point to the superolateral angle of the patella (cm) | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | F/54 | Left | Malignant fibrous histiocytoma | Anteromedial knee | SCP | 15×8 | 19 | 40 | 12 | Survival |
| 2 | F/45 | Right | Post-burn scar contracture | Anteroinferior knee | MCP | 24×8 | 22 | 38 | 14 | Survival |
| 3 | M/11 | Left | Post-burn scar contracture | Anteroinferior knee | MCP | 16×8 | 17 | 34 | 11 | Survival |
| 4 | F/13 | Right | Angiofibrolipoma | Anteroinferior knee | SCP | 9×7 | 19 | 30 | 12 | Survival |
| 5 | M/3 | Left | Post-burn scar contracture | Posterolateral thigh | MCP | 15×6 | 15 | 22 | 10 | Survival |
| 6 | M/58 | Left | Soft-tissue sarcoma | Anteromedial knee | MCP | 15×7 | 12 | 44 | 14 | Survival |
| 7 | M/54 | Right | Post-burn scar contracture | Lateral knee | MCP | 20×7 | 20 | 46 | 11 | Survival† |
F, female; M, male; SCP, septocutaneous perforator; MCP, musculocutaneous perforator; AP line, a line is drawn between the anterior superior iliac spine and the upper outer border of the patella. †, free flap
Surgical technique
The ALT flap was designed following a well-described conventional pattern (5,6). The patient was first placed in a supine position. A line was then drawn connecting the anterior superior iliac spine and the superolateral border of the patella (AP line), which roughly corresponded to the intermuscular septum between the rectus femoris and the vastus lateralis muscles. The midpoint of this line was also marked. A handheld ultrasound Doppler was used to thoroughly explore the perforators supplying the ALT flap along the AP line, particularly around the line midpoint. The initial design of the flap was then marked to indicate these perforators.
A medial incision was first made down to the deep fascia above the rectus femoris muscle. Subfascial dissection then proceeded laterally until the intermuscular septum was reached. We preferred to open the septum in a distal to proximal direction. Care should be taken not to compromise the potential septocutaneous perforators that traverse the septum. Once the septum was adequately opened, the overall characteristics of the LCFA descending branch could be visualized.
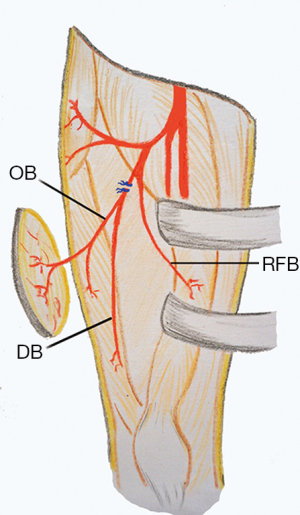
If an oblique branch originating from the LCFA descending branch was present, it could be seen as a vessel lying laterally to the descending branch. If the vessel supplying the flap from the oblique branch was a septocutaneous perforator, it could be easily dissected out up to point of origination from the descending branch (Figure 1). For musculocutaneous perforators, intramuscular dissection was needed to completely isolate the oblique branch (Figure 2). The descending branch proximal to the origin of the oblique branch was then ligated and cut, and the pedicle was distally dissected to the point where the descending branch entered the vastus lateralis muscle. This point might serve as the pivot point. The descending branch was normally not dissected within the vastus lateralis muscle. The main motor nerve servicing the vastus lateralis muscle was carefully preserved during the surgery. The flap was then raised completely and transferred to the recipient site through a subcutaneous tunnel or an open wound (Figure 3).


The donor site was primarily closed in layers over closed suction drains. Postoperatively, the skin paddle was monitored for changes in color, turgor, and temperature without specialized instruments. No effort was made to identify or monitor a perforator using Doppler. The donor thigh was monitored for signs of neurovascular injury and compartment syndrome. The sutures were removed 14 days postoperatively and the patients were encouraged to walk thereafter.
Results
Seven dALT flaps were transferred in 7 patients and all flaps survived completely without any major complications. Oblique branches in these cases originated from the descending branch in 6 patients and transverse branch in 1 patient. The perforators on which the flaps were based included five musculocutaneous perforators and two septocutaneous perforators. Preoperative computed tomographic angiography was performed on three patients. The average flap size was 16.3 cm long × 7.3 cm wide (range, 9–24×6–8 cm), while the mean pedicle length was 17.7 cm (range, 12–22 cm). The mean length of the AP line was 36.3 cm (range, 22–46 cm) and the mean distance from the flap pivot point to the superolateral border of the patella was 12 cm (range,10–14 cm).
In one case (case 1), the distal portion of the LCFA descending branch, particularly the arterial component, was severely hypoplastic and thus could not be used as a reverse-flow pedicle for the dALT flap. In this patient, we found the rectus femoris branch originated from the descending branch at a position proximal to the oblique branch. The dALT flap was then successfully raised based on the reverse flow from the rectus femoris branch through the rectus femoris muscle. In another case (case 7), no sizable perforators were found in the ALT region from the descending branch and meanwhile the oblique branch originated from the LCFA transverse branch. Therefore, the oblique and descending branches had to be ligated and cut. The pedicled dALT flap was then converted to a free flap with the oblique branch anastomosed to the descending branch in an end-to-end fashion. The microsurgical flap survived completely.
The donor site could be primarily closed in all patients. No major complications, such as wound dehiscence or infection, occurred. The patients were encouraged to walk 14 days postoperatively after suture removal. The patients showed no signs of tumor recurrence or muscle weakness after a mean follow-up period of 28.7 months (range, 2–48 months).
Discussion
Traditionally, the LCFA descending branch serves as the pedicle for ALT flaps (2). The perforators supplying the ALT skin may arise from source vessels other than the LCFA descending branch (5,6). Wong et al. (4) defined a previously unnamed vascular branch in the lateral thigh as the oblique branch of the LCFA. In a prospective analysis of 89 consecutive flaps, they noted the presence of an oblique branch in the lateral thigh in 34% of patients. When present, the oblique branch variably supplies blood to the vastus lateralis muscle and ALT skin. The origin of the oblique branch itself is variable. It may arise from the descending branch (36%), transverse branch (52%), or the LCFA (6%). In rare cases, the oblique branch may even arise directly from the profunda femoris (3%) or femoral artery (3%). Wong et al. also demonstrated that the oblique branch can reliably be used as the pedicle of either an ALT perforator or musculocutaneous flaps. Of the 89 patients in that study, 14% of ALT flaps were based on the oblique branch, leaving the descending branch in situ (4). When present, the oblique branch is very sizable and consists of an artery and usually two veins. Wong et al. showed that the pedicle of the ALT flap can be served by either the descending or oblique branches. However, some authors questioned whether the oblique branch indeed exists and speculated that this branch could in fact be the low-lying transverse branch of the LCFA (7). Nevertheless, the feasibility of using the oblique branch in ALT flap procedures, either as a pedicle or a free flap, has been demonstrated (4,8-10).
The clinical significance of the LCFA oblique branch is summarized as follow. First, when raising an ALT myocutaneous flap, surgeons should consider that the ALT skin may be supplied exclusively by vessels from the oblique branch rather than the descending branch. Otherwise, although a large block of the vastus lateralis muscle is included in the myocutaneous flap, the cutaneous component would be necrotic (4,11,12). Wong et al. (8) proposed a modified approach to harvest an ALT myocutaneous flap to safeguard against such anatomic variations. They suggested that the intermuscular septum between the vastus lateralis and rectus femoris muscles first be opened. Before transection of the vastus lateralis muscle, one or two of the most sizable perforators are traced proximally by removing the muscle or septum over the perforators to clearly reveal the precise origin of these perforators. If the cutaneous perforators originate from the LCFA descending branch, then the ALT myocutaneous flap can be elevated in a conventional way. If the cutaneous perforators directly originate from the oblique branch and converge with the descending branch to form a common trunk, the latter can be used as the flap pedicle. If the oblique and descending branches originate from different source vessels that separately nourish the ALT skin and vastus lateralis muscle, then the myocutaneous flap should be based on these two branches and two sets of microvascular anastomoses might be needed. Second, when raising an ALT cutaneous or myocutaneous flap, if the oblique branch originates from the descending branch and a much longer pedicle is not needed, the flap might be based only on the oblique branch with the descending branch left in situ, such that anterolateral thigh musculature devascularization would be minimized (4). Third, a chimeric ALT flap is a useful option for reconstructing complex defects that involve various tissue types. The design of a chimeric ALT flap would be more versatile if the LCFA oblique branch is included as a potential source vessel that supplies the skin and vastus lateralis muscle (10). Fourth, for reconstructing defects using microsurgical techniques when recipient site vessels cannot be sacrificed or require repair, a flow-through ALT flap is a commonly used option for defect reconstruction and simultaneously spares the recipient site vessels (13). If an LCFA oblique branch is present and arises from the descending branch, a flow-through ALT flap may be raised based on the oblique branch. In this situation, the oblique branch originates from the descending branch more proximally than other perforators from the descending branch. Therefore, a segment of the descending branch with a relatively large caliber may be harvested to bridge the recipient site vessel. Fifth, defect reconstruction using a microvascular flap in the thigh still presents a surgical challenge, partly due to a lack of suitable recipient vessels. The LCFA descending branch, in either an antegrade-flow or a retrograde-flow fashion, has been used as a microsurgical recipient vessel (14,15). When the LCFA oblique branch presents with a sizable caliber, it might thus serve as a potential recipient vessel for microanastomosis. Finally, the pedicled ALT flap, either proximally- or distally-based, can be used to reconstruct defects from the abdomen to the proximal portion of the leg. A proximally-based ALT may be raised based on the oblique branch without compromising the LCFA descending branch, while a distally-based ALT flap based on the oblique branch can also be raised as discussed below.
The distally-based ALT (dALT) flap is a useful option to reconstruct defects around the knee (1,3). Although the pertinent anatomical basis of this flap has seemingly been well documented, to the best of our knowledge, there are no reports describing the significance of the LCFA oblique branch in raising a dALT flap. Among the 18 patients in our study who underwent defect reconstruction using dALT flaps, seven flaps (38.9%) were based on the oblique branch from the LCFA descending branch. We found that the frequency of a septocutaneous perforator originating from the oblique branch is higher than that from the descending branch. Even if a musculocutaneous perforator is present, it is often covered by only a small amount of muscle, such that intramuscular dissection and uncovering of the perforator would be relatively easy and straightforward (4). Some authors once referred to this type of musculocutaneous perforator as a musculoseptocutaneous (or semi-septocutaneous) perforator (16). Through clinical observation, we found that the origin of the oblique branch in the descending branch lies distal to the rectus femoris branch. Therefore, the oblique branch may be ligated and cut distal to the originating point of the rectus femoris branch in the descending branch. Devascularization of the rectus femoris muscle and subsequent muscle necrosis is avoided. Moreover, the origin of the oblique branch is more proximal than that of the low-lying perforators from the descending branch. Therefore, a much longer pedicle can be obtained and the reach of the flap can be significantly increased.
Anatomic variations and absence of the oblique branch constitute the main drawback of this technique. The oblique branch from the LCFA descending branch was identified in 38.9% of our cases, which is similar to the frequency reported by Wong et al. (35.5%) (4). As such, preoperative computed tomography angiography is highly recommended to evaluate the overall configuration of the LCFA system and ensure a safe and feasible operation. If the oblique branch originates from the LCFA transverse branch, a dALT flap can still be harvested, although extensive devascularization of the ALT muscles may occur after ligation of the corresponding muscular branches. If the oblique branch originates from source vessels other than the LCFA system, a dALT flap cannot be raised as usual. In these two situations, surgeons may have two options: (I) the flap can be raised based on the perforators from the LCFA descending branch in a traditional fashion; or (II) the pedicled dALT flap can be converted to a free flap wherein the oblique and descending branches are ligated, cut proximally, and anastomosed to each. Many authors have observed an inverse relationship (17) or perforator dominance phenomenon (18) between adjacent angiosomes and perforator territories. We found that the oblique branch from the descending branch can be subdivided into at least two types: an oblique branch with or without a robust vastus lateralis muscular branch. If an oblique branch sending sizable perforators to the ALT skin has a robust muscular branch, then the cutaneous perforators and muscular branches arising from the descending branch might be small in caliber, or even absent, and vice versa. However, the relationship between the cutaneous perforators and muscular branches of the oblique and descending branches requires further investigation.
Conclusions
A dALT flap can be reliably raised based on the oblique branch when it arises from the LCFA descending branch and sends cutaneous perforators to the ALT region. The dissection of the perforator is simple and straightforward and the pedicle length is much longer than that of flaps based on perforators originating from the LCFA descending branch.
Acknowledgments
The parents and patients who participated in this study are gratefully acknowledged.
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Geoffrey Hallock, Juyu Tang) for the series “Perforator Flap” published in Journal of Xiangya Medicine. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jxym.2018.04.03). The series “Perforator Flap” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures were approved by the Institutional Review Board of the Chinese Academy of Medical Science and Peking Union Medical College prior to the execution of the study. Patients and/or direct family members gave written consent to the procedure. The present study strictly abides by the Declaration of Helsinki (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Zhang GL. Reversed anterolateral thigh island flap and myocutaneous flap transplantation. Zhonghua Yi Xue Za Zhi 1990;70:676-8, 46.
- Xu DC, Zhong SZ, Kong JM, et al. Applied anatomy of the anterolateral femoral flap. Plast Reconstr Surg 1988;82:305-10. [Crossref] [PubMed]
- Pan SC, Yu JC, Shieh SJ, et al. Distally based anterolateral thigh flap: an anatomic and clinical study. Plast Reconstr Surg 2004;114:1768-75. [Crossref] [PubMed]
- Wong CH, Wei FC, Fu B, et al. Alternative vascular pedicle of the anterolateral thigh flap: the oblique branch of the lateral circumflex femoral artery. Plast Reconstr Surg 2009;123:571-77. [Crossref] [PubMed]
- Kimata Y, Uchiyama K, Ebihara S, et al. Anatomic variations and technical problems of the anterolateral thigh flap: a report of 74 cases. Plast Reconstr Surg 1998;102:1517-23. [Crossref] [PubMed]
- Shieh SJ, Chiu HY, Yu JC, et al. Free anterolateral thigh flap for reconstruction of head and neck defects following cancer ablation. Plast Reconstr Surg 2000;105:2349-57. [Crossref] [PubMed]
- Hubmer MG, Feigl G. Alternative vascular pedicle of the anterolateral thigh flap: does an oblique branch really exist? Plast Reconstr Surg 2010;125:1580-1; author reply 1581. [Crossref] [PubMed]
- Wong CH, Kao HK, Fu B, et al. A cautionary point in the harvest of the anterolateral thigh myocutaneous flap. Ann Plast Surg 2009;62:637-9. [Crossref] [PubMed]
- Wong CH, Ong YS, Wei FC. The anterolateral thigh - Vastus lateralis conjoint flap for complex defects of the lower limb. J Plast Reconstr Aesthet Surg 2012;65:235-9. [Crossref] [PubMed]
- Liu WW, Guo ZM. Reconstruction of wide-apart double defect using a branch-based chimeric anterolateral thigh flap. Plast Reconstr Surg Glob Open 2014;2:e96. [Crossref] [PubMed]
- da Costa AC, Lancelotti CL. Oblique branch of the lateral circumflex femoral artery also found in 32 percent of cadavers in Brazil. Plast Reconstr Surg 2009;124:1011-2; author reply 1012-3. [Crossref] [PubMed]
- Wong CH. The oblique branch trap in the harvest of the anterolateral thigh myocutaneous flap. Microsurgery 2012;32:631-4. [Crossref] [PubMed]
- Qing L, Wu P, Liang J, et al. Use of flow-through anterolateral thigh perforator flaps in reconstruction of complex extremity defects. J Reconstr Microsurg 2015;31:571-8. [Crossref] [PubMed]
- Hallock GG. The vascular pedicle of the anterolateral thigh flap as an alternative recipient site for thigh free flaps. J Reconstr Microsurg 2008;24:131-6. [Crossref] [PubMed]
- Gao SH, Feng SM, Chen C, et al. A new recipient artery for reconstruction of soft-tissue defects in the lower limb with a free anterolateral thigh flap: the reversed descending branch of the lateral femoral circumflex artery. Plast Reconstr Surg 2012;130:1059-65. [Crossref] [PubMed]
- Lakhiani C, Lee MR, Saint-Cyr M. Vascular anatomy of the anterolateral thigh flap: a systematic review. Plast Reconstr Surg 2012;130:1254-68. [Crossref] [PubMed]
- Yu P. Inverse relationship of the anterolateral and anteromedial thigh flap perforator anatomy. J Reconstr Microsurg 2014;30:463-8. [Crossref] [PubMed]
- Rozen WM, Grinsell D, Koshima I, et al. Dominance between angiosome and perforator territories: a new anatomical model for design of perforator flaps. J Reconstr Microsurg 2010;26:539-45. [Crossref] [PubMed]
Cite this article as: Liu Y, Zang M, Zhu S, Chen B, Li S, Xue B, Xie T. Distally based anterolateral thigh flap pedicled on the oblique branch of the lateral circumflex femoral artery. J Xiangya Med 2018;3:17.


