Dynamic infrared mapping of cutaneous perforators
Introduction
Flap design has evolved constantly over the last decades, culminating with the introduction of flaps harvested on a single perforator back in 1989 (1). Since then, their use has grown exponentially, perforator flaps having replaced axial and musculocutaneous flaps in the vast majority of reconstructive procedures. However, these flaps are a lot harder to harvest, much of the difficulty being attributable to correctly identifying and dissecting the ideal perforator in each case. Therefore, preoperative perforator mapping has become an integral part of the reconstructive protocol in many institutions (2).
Out of the numerous imaging modalities used for perforator mapping, handheld Doppler ultra-sound, color Doppler imaging, computed tomography (CT) angiography, and MRI are worth mentioning. Currently, CT Angiography is considered the “gold standard”, providing reliable results, fast and with minimal patient discomfort (3). However, all these techniques have drawbacks, whether its exposure to radiation, high costs or operator dependence.
Dynamic infrared thermography (DIRT) is a fairly new imaging technique, showing promising results, in preoperative perforator mapping, intraoperative perfusion assessment and postoperative flap monitoring (4). The technique is based on visualizing the infrared radiation (IR) emitted by the skin, and correlating it with tissue temperature. The mapping technique was first described by Itoh and Arai (5), and then de Weerd (6) in 2006, followed by a series of studies by Chubb and Taylor (7) that emphasize the value of thermography not only for perforator mapping but also for identifying the connections between perforasomes. DIRT is a safe, fast, noninvasive, cost effective and reliable method of perforator mapping.
Basic science
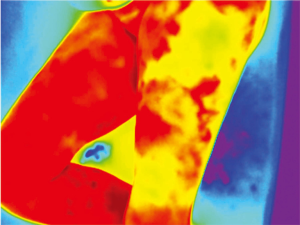
The human body emits heat in the form of IR. The IR wavelength is a part of the non-visible electromagnetic spectrum (700 nm – 1 mm) and is perceived as heat. The amount of IR radiation emitted is determined by the temperature of the skin (8). Because cutaneous temperature depends on vascularization (9), IR skin emission mirrors its blood supply. Older thermal cameras were cumbersome and had a low thermal sensitivity, without the capacity to perceive subtle differences in temperature. The new generation of thermal cameras, however, have a thermal sensitivity of <40 mk and can pick-up differences of 0.04 °C (10). This, plus advances in image post-processing software, has reignited the interest for medical thermography. The IR recorded images are processed by the camera and displayed as a color-coded map. A “rainbow” color scheme is normally used, higher temperatures appearing as red, while lower temperatures are depicted in blue. This allows for a very simple interpretation of the data, making the technique straightforward and operator friendly.
Equipment and technique
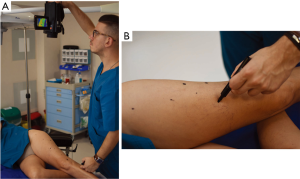
The perforator mapping can be done the day before or in the operating room just before the procedure. The equipment required for DIRT is simple and relatively non-expensive, when compared with other imaging modalities. All that is required is a thermal camera (Figure 1) [Testo 890 (Testo AG, Lenzkirch, Germany)] and any machine capable of blowing cold air, for example a fan (Figure 2). The idea behind dynamic thermography mapping of perforators relies on recording the thermal recovery of the skin after an induced cold challenge.

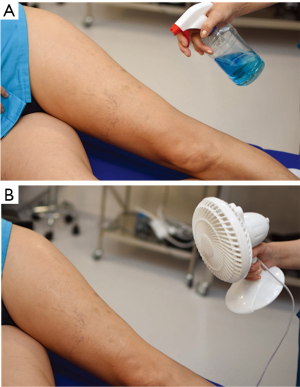
After the flap is outlined, an alcohol based solution is sprayed and cold air is blown on the skin surface for a couple of minutes (≅3 min), until the area is evenly cooled to a temperature well below that of the normal skin, roughly 20 °C. The temperature is monitored with the thermal camera. Once the temperature is low enough, the cooling stops and the flap is allowed to rewarm (11).
Because of skin vascularization through perforators, as the temperature recovers, they are visualized first. The rewarming phase is monitored in real time through the thermography camera, recording the initial bright spots that appear, or “hot spots”, and marking them as perforators (Figure 3). The sequence of hot spots visualization and signal intensity mirrors the skin vascular supply. The bigger, “dominant” perforators, always appear first and generate a stronger signal (12). This also indicates a well-developed vascular network around the perforator. After all the perforators are marked, the thermography is repeated, confirming the location of the dominant perforator (Figure 4). The sequence of expected visible color changes is shown in Figures 5-9. A final overlay of the thermographic image on the normal skin where perforators have been marked will confirm their location (Figure 10).
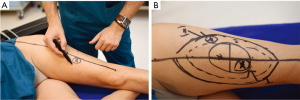
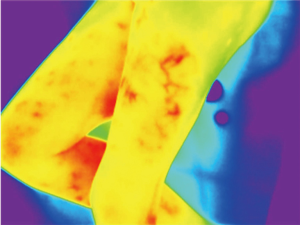
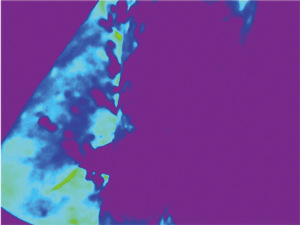
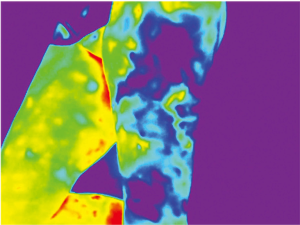
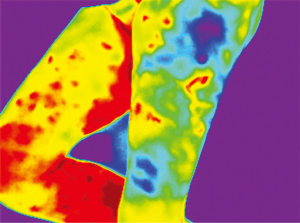
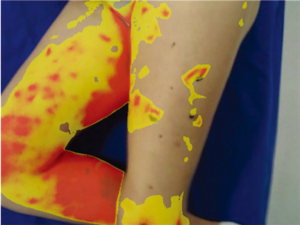
Thermography identifies the perforators superficially, as they enter the skin. If the perforator is compared to a tree, thermography only visualizes the “crown”. For this reason, it is possible to record multiple “hot spots” for one perforator, because perforators occasionally branch. For this same reason, as some perforators have a slightly oblique course from the fascia to the skin, their registered “hot spot” may deviate from the actual fascial emergence. However, according to studies carried out by Giunta et al. the average of this deviation is about 8 mm (13).
Conclusions
DIRT mapping of cutaneous perforators is a fast, reliable, safe and non-expensive method of perforator mapping. It does not employ contrast material or radiation. The examination technique is simple, easy to learn and operator friendly. However, it is a subjective test that requires operator experience for accuracy. It can be used as a stand-alone mapping tool or in adjunct with other techniques such as audible Doppler ultrasound (14). DIRT delivers accurate and reproducible results when compared CTA (15) and the audible Doppler, without the drawbacks associated with these techniques. Further research into this emerging field will establish the place of DIRT in the modern plastic surgery armamentarium.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Geoffrey Hallock, Juyu Tang) for the series “Perforator Flap” published in Journal of Xiangya Medicine. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jxym.2018.04.05). The series “Perforator Flap” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflict of interests to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Koshima I, Soeda S. Inferior epigastric artery skin flaps without rectus abdominis muscle. Br J Plast Surg 1989;42:645-8. [Crossref] [PubMed]
- Masia J, Clavero JA, Larrañaga J, et al. Preoperative planning of the abdominal perforator flap with multidetector row computed tomography: 3 years of experience. Plast Reconstr Surg 2008;122:80e-1e. [Crossref] [PubMed]
- Uppal RS, Casaer B, Van Landuyt K, et al. The efficacy of preoperative mapping of perforators in reducing operative times and complications in perforator flap breast reconstruction. J Plast Reconstr Aesthet Surg 2009;62:859-64. [Crossref] [PubMed]
- Muntean MV, Muntean V, Ardelean F, et al. Dynamic perfusion assessment during perforator flap surgery: an up-to-date. Clujul Med 2015;88:293-7. [Crossref] [PubMed]
- Itoh Y, Arai K. Use of recovery-enhanced thermography to localize cutaneous perforators. Ann Plast Surg 1995;34:507-11. [Crossref] [PubMed]
- de Weerd L, Mercer JB, Setså LB. Intraoperative dynamic infrared thermography and free-flap surgery. Ann Plast Surg 2006;57:279-84. [Crossref] [PubMed]
- Chubb DP, Taylor GI, Ashton MW. True and 'choke' anastomoses between perforator angiosomes: part II. dynamic thermographic identification. Plast Reconstr Surg 2013;132:1457-64. [Crossref] [PubMed]
- Jones BF. A reappraisal of the use of infrared thermal image analysis in medicine. IEEE Trans Med Imaging 1998;17:1019-27. [Crossref] [PubMed]
- Awwad AM, White RJ, Webster MH, et al. The effect of temperature on blood flow in island and free skin flaps: an experimental study. Br J Plast Surg 1983;36:373-82. [Crossref] [PubMed]
- Jiang LJ, Ng EY, Yeo AC, et al. A perspective on medical infrared imaging. J Med Eng Technol 2005;29:257-67. [Crossref] [PubMed]
- Taylor GI, Chubb DP, Ashton MW. True and 'choke' anastomoses between perforator angiosomes: part i. anatomical location. Plast Reconstr Surg 2013;132:1447-56. [PubMed]
- de Weerd L, Miland AO, Mercer JB. Perfusion dynamics of free DIEP and SIEA flaps during the first postoperative week monitored with dynamic infrared thermography. Ann Plast Surg 2009;62:42-7. [Crossref] [PubMed]
- Giunta RE, Geisweid A, Feller AM. The value of preoperative Doppler sonography for planning free perforator flaps. Plast Reconstr Surg 2000;105:2381-6. [Crossref] [PubMed]
- Muntean MV, Strilciuc S, Ardelean F, et al. Using dynamic infrared thermography to optimize color Doppler ultrasound mapping of cutaneous perforators. Med Ultrason 2015;17:503-8. [PubMed]
- de Weerd L, Weum S, Mercer JB. The value of dynamic infrared thermography (DIRT) in perforatorselection and planning of free DIEP flaps. Ann Plast Surg 2009;63:274-9. [Crossref] [PubMed]
Cite this article as: Muntean MV, Strilciuc S, Ardelean F, Georgescu AV. Dynamic infrared mapping of cutaneous perforators. J Xiangya Med 2018;3:16.