
Tissue expansion for improvement of the perforator flap donor site
Introduction
A perforasome (1) or skin territory supplied by a specific perforator can be selected using any of the 390 major perforators found on average in everyone (2). This means that the donor site of a perforator flap can now be selected so as to best match the characteristics of the recipient site, i.e., as regards color, bulk, durability, texture, etc.; as long as the requisite perforator exists. Since the choice of perforator flap is so readily available, further improvements in the selection process have concentrated on minimizing any donor site residue. Not only must donor site function be preserved, but the resulting deformity should be as inconspicuous as possible. Because muscle never need be included with a perforator flap, maximum function by definition is always retained. The goal for the donor site then should be to obtain the shortest possible linear scar.
Simple primary closure of the perforator flap donor site may not always be possible, especially when too large to allow this. A skin graft will then always be a solution; but is at the best non-aesthetic, and may cause later problems with contractures, adhesions limiting motion of the underlying muscles (3) or retardation of growth in children (4,5), or muscle bulging after a subfascial dissection causing a contour deformity (6). Attempted closure under excessive tension just to avoid a skin graft can cause a disastrous compartment syndrome (7). Even if a suprafascial harvest were done, fascial imbrication alone may still sufficiently narrow the donor site to allow skin approximation (8). Recently, automatic or self-tightening continuous external tissue expanders attached to the wound margins, by skin stretching will gradually over time allow a direct closure (DermaClose®, Synovis/Micro Companies Alliance, Birmingham, Alabama, USA) (9), but are expensive if even available. Synchronous transposition of adjacent tissue flaps can also avoid a skin graft (10-13), yet require the morbidity of additional dissection and resultant greater scar length. Narrow split chimeric perforator flaps can be placed side by side to increase the needed total flap surface area, while permitting direct donor site closure (14-16), albeit rarely possible since multiple closely aligned major perforators must exist.
Another consideration to minimize the deformity, especially in the absence of other major perforators near the donor site, is tissue expansion. Tissue expansion, e.g., as a sequela of pregnancy, is an age old (no pun intended) and well known phenomenon. Whether the increased skin surface area achieved is actually due to the creation of a net tissue gain or just a consequence of acute load cycling remains controversial (17,18). This capability has been used successfully for free flap donor sites including perforator flaps for decades (19-22), as well as more recently for pedicled perforator flaps (23,24).
Methods
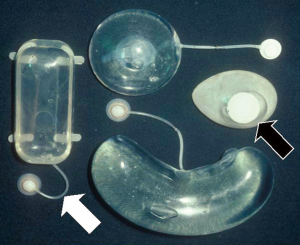
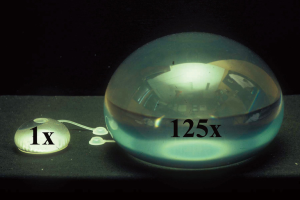
To initiate tissue expansion, first an expander device must be placed under the skin chosen to be expanded. This can follow three formats with little variation dependent on the timing of the perforator flap harvest, i.e., pre-transfer, concurrent, or post-transfer (25). A typical expander envelope has a silicone shell with an intrinsic or extrinsic one-way valve into which saline is injected to inflate the balloon (Figure 1). The extrinsic valve is connected to the envelope via a long tube, so that it can be placed far from the implant pocket to avoid the risk of inadvertent shell puncture by the injection needle, particularly a problem by the time significant expansion has been achieved. A new needleless expander using intrinsic compressed carbon dioxide gas as the medium rather than saline is available, but so far only used for breast reconstruction (26). Although conventional expanders come in various shapes and sizes (Figure 1), the rectangular design is the most useful. The size needed often depends on the location and availability of skin to be expanded. As large as possible a rectangle expander, and even multiple if necessary, are suggested; but compensation if using a smaller implant is possible, as everyone can be expanded many fold beyond the stated vendor’s capacity before risk of rupture (Figure 2) (27).
Pre-transfer expansion
The design of the potential perforator flap is marked about the specified perforator that has been identified using customary approaches. A pocket for placement of the expander must not be near that perforator; not just to insure that it is not injured during pocket creation, but because of the expectation of an inflammatory response and scarring caused by the expansion itself that can make later dissection of the perforator unnecessarily difficult (21). The expander envelope itself will serve as a template to determine the subcutaneous area involved that will best accept it without wrinkling. An incision away from and perpendicular to a border of the designed flap will best allow formation of the desired size pocket above it, most simply under direct visualization, with concomitant placement of the external valve below and far enough away to prevent accidental envelope puncture while still readily palpable for easy needle insertion later.
Synchronous expansion
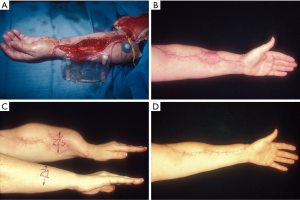
Concurrent with flap harvest, a suprafascial pocket beneath the subcutaneous tissues is made starting from the edge of the open wound remaining after the flap is removed (Figure 3). This should extend slightly beyond the dimensions of the expander to insure again that there is neither wrinkling nor chance that the edge of the envelope is near the donor site that must be skin grafted. The valve must also be placed well beyond both the implant and the wound border. At least a two layer closure is then mandatory by first bringing the subcutaneous tissues down to the fascia, and then the dermis itself to the fascia beyond that using horizontal half-buried mattress sutures. All evidence of the subcutaneous tissues must be hidden to insure that if one layer dehisces, the implant will not be inadvertently exposed while the donor site skin graft heals to seal the site of placement.
Post-transfer expansion
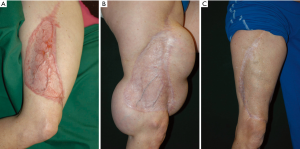
For a healed and usually skin grafted perforator flap donor site, the required number of expanders can be placed at strategic points about it (Figure 4). The approach will be identical to that of pre-transfer expansion, except that the incision made should never be at or near the junction of the skin graft and native skin. That site typically would heal poorly or dehisce readily once expansion begins, with implant exposure.
Technique
A conservative approach before commencing expansion is reasonable. Usually this is within 2 weeks of envelope placement, but never before all incisions or skin graft junctions seem to be adequately healing. If incremental saline injections are chosen, the best way to determine the safe fill volume to instill is when the patient notes discomfort (28). Lack of capillary refill over the expander also should be a concern, especially in the stoic patient. Elaborate monitoring methods such as transcutaneous tissue oxygen measurements are accurate, but no more sensitive than the onset of pain (28). The frequency of instillations again depends on the equipment utilized, but the patient should be monitored for untoward events at least periodically.
The endpoint of expansion can be deceptive. For an extremity, measurement of the ipsilateral expanded circumference must be slightly greater than the sum of the contralateral extremity circumference at the same level plus the width of the skin grafted defect (Figure 3). For non-extremity defects, the distance overlying the expander gained is estimated as well as possible. In either circumstance, any potential for more expanded skin is always preferable.
At the time of perforator flap harvest or skin graft deformity removal, the edges of the wound so created are used to retrieve the implant and valve. Maximum injection of saline into the valve just before this step may gain a centimeter or so of acute skin stretch. Donor site skin closure without tension is then done; but if that is encountered, it is always wisest to leave some skin graft and come back later to serial excise it.
Discussion
There are many different ways to achieve tissue expansion with many different devices and techniques. Use what method one becomes comfortable with. Expanders, for example, could be placed submuscularly which automatically assures that musculocutaneous perforators are never violated. They can also be placed subfascially, which is an easily dissected and relatively bloodless plane. However, in both these situations, restricted stretching and subsequent expansion can be more difficult and painful.
Complications during expansion can be expected and best avoided. As already stressed, wound dehiscence with implant or valve exposure or extrusion must be rectified promptly as otherwise infection is inevitable. Valves and implants can both leak leading to deflation, and usually are related to needle injections (29). Overfilling can result in ischemia and skin necrosis. Complications after the completion of expansion include overzealous wound closure attempts unlikely leading to a compartment syndrome, but more likely to wound edge skin necrosis and dehiscence with delayed healing.
The choice of tissue expansion requires at the least the inconvenience of 2 surgical procedures—implant in and implant out. Depending on the system, frequent outpatient clinical visits are required. This is usually a long process, extending over months until expansion is deemed adequate (25). Both the patient and the surgeon must be patient and compliant. This will be easier for those undergoing pre-transfer or post-transfer expansion, which will be basically an elective and well informed choice that is not possible with the synchronous group where there has not been enough time to develop any meaningful rapport. Tissue expansion must be recognized not to be a panacea for solving all dilemmas for closure of perforator flap donor sites, but is one tested option of many with a long history that must be considered.
Acknowledgments
Thanks to David C. Rice, B.S., P.E., Sacred Heart Hospital, Allentown, Pennsylvania, who assisted with the microsurgical aspects.
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Journal of Xiangya Medicine for the series “Perforator Flap”. The article has undergone external peer review.
Conflicts of Interest: The author has completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jxym.2018.03.02). The series “Perforator Flap” was commissioned by the editorial office without any funding or sponsorship. GH served as an unpaid Guest Editor of the series and serves as an unpaid editorial board member of Journal of Xiangya Medicine from Apr 2017 to Mar 2019. The author has no other conflicts of interest to declare.
Ethical Statement: The author is accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Saint-Cyr M, Wong C, Schaverien M, et al. The perforasome theory: vascular anatomy and clinical implications. Plast Reconstr Surg 2009;124:1529-44. [Crossref] [PubMed]
- Taylor GI, Palmer JH. The vascular territories (angiosomes) of the body: experimental study and clinical applications. Br J Plast Surg 1987;40:113-41. [Crossref] [PubMed]
- Kimata Y, Uchiyama K, Ebihara S, et al. Anterolateral thigh flap donor-site complications and morbidity. Plast Reconstr Surg 2000;106:584-9. [Crossref] [PubMed]
- Zhao Y, Qiao Q, Liu Z, et al. Alternative method to improve the repair of the donor site of the anterolateral thigh flap. Ann Plast Surg 2002;49:593-8. [Crossref] [PubMed]
- Novak CB, Lipa JE, Noria S, et al. Comparison of anterolateral thigh and radial forearm free flap donor site morbidity. Microsurgery 2007;27:651-4. [Crossref] [PubMed]
- Lipa JE, Novak CB, Binhammer PA. Patient-reported donor-site morbidity following anterolateral thigh free flaps. J Reconstr Microsurg 2005;21:365-70. [Crossref] [PubMed]
- Addison PD, Lannon D, Neligan PC. Compartment syndrome after closure of the anterolateral thigh flap donor site: a report of two cases. Ann Plast Surg 2008;60:635-8. [Crossref] [PubMed]
- Rodriguez ED, Bluebond-Langner R, Park J, et al. Does fascia lata repair facilitate closure and does it affect compartment pressures of the anterolateral thigh flap donor site? Plast Reconstr Surg 2007;120:1300-4. [Crossref] [PubMed]
- Senchenkov A, Agag RL, Lee J, et al. Management of anterolateral thigh free flap donor site defects with a continuous external tissue expander. Microsurgery 2015;35:290-4. [Crossref] [PubMed]
- Yamada N, Kakibuchi M, Kitayoshi H, et al. A new way of elevating the anterolateral thigh flap. Plast Reconstr Surg 2001;108:1677-82. [Crossref] [PubMed]
- Hanasono MM, Skoracki RJ, Yu P. A prospective study of donor-site morbidity after anterolateral thigh fasciocutaneous and myocutaneous free flap harvest in 220 patients. Plast Reconstr Surg 2010;125:209-14. [Crossref] [PubMed]
- Calderón W, Borel C, Roco H, et al. Primary closure of donor site in anterolateral cutaneous thigh free flap. Plast Reconstr Surg 2006;117:2528-9. [Crossref] [PubMed]
- Hallock GG. Closure of the ulnar forearm free flap donor site using a local radial forearm flap. Br J Plast Surg 1992;45:94-6. [Crossref] [PubMed]
- Marsh DJ, Chana JS. Reconstruction of very large defects: a novel application of the double skin paddle anterolateral thigh flap design provides for primary donor-site closure. J Plast Reconstr Aesthet Surg 2010;63:120-5. [Crossref] [PubMed]
- Sano K, Hallock GG, Hamazaki M, et al. The perforator-based conjoint (chimeric) medial Sural(MEDIAL GASTROCNEMIUS) free flap. Ann Plast Surg 2004;53:588-92. [Crossref] [PubMed]
- Zhang YX, Hayakawa TJ, Levin LS, et al. The Economy in Autologous Tissue Transfer: Part 1. The Kiss Flap Technique. Plast Reconstr Surg 2016;137:1018-30. [Crossref] [PubMed]
- Austad ED, Thomas SB, Pasyk K. Tissue expansion: dividend or loan? Plast Reconstr Surg 1986;78:63-7. [Crossref] [PubMed]
- Hirshowitz B, Kaufman T, Ullman J. Reconstruction of the tip of the nose and ala by load cycling of the nasal skin and harnessing of extra skin. Plast Reconstr Surg 1986;77:316-21. [Crossref] [PubMed]
- Hallock GG. Free flap donor site refinement using tissue expansion. Ann Plast Surg 1988;20:566-72. [Crossref] [PubMed]
- Leighton WD, Russell RC, Feller AM, et al. Experimental pretransfer expansion of free-flap donor sites: II. Physiology, histology, and clinical correlation. Plast Reconstr Surg 1988;82:76-87. [Crossref] [PubMed]
- Hallock GG. The preexpanded anterolateral thigh free flap. Ann Plast Surg 2004;53:170-3. [Crossref] [PubMed]
- Hallock GG. Refinement of the radial forearm flap donor site using skin expansion. Plast Reconstr Surg 1988;81:21-5. [Crossref] [PubMed]
- Kulahci Y, Sever C, Uygur F, et al. Pre-expanded pedicled thoracodorsal artery perforator flap for postburn axillary contracture reconstruction. Microsurgery 2011;31:26-31. [Crossref] [PubMed]
- Sever C, Kulahci Y, Eren F, et al. Reconstruction of postburn cervical contractures using expanded supraclavicular artery flap. J Burn Care Res 2013;34:e221-7. [Crossref] [PubMed]
- Hallock GG. Tissue expansion techniques to minimize morbidity of the anterolateral thigh perforator flap donor site. J Reconstr Microsurg 2013;29:565-70. [Crossref] [PubMed]
- Ascherman JA, Zeidler K, Morrison KA, et al. Carbon Dioxide-Based versus Saline Tissue Expansion for Breast Reconstruction: Results of the XPAND Prospective, Randomized Clinical Trial. Plast Reconstr Surg 2016;138:1161-70. [Crossref] [PubMed]
- Hallock GG. Maximum overinflation of tissue expanders. Plast Reconstr Surg 1987;80:567-9. [Crossref] [PubMed]
- Hallock GG, Rice DC. Objective monitoring for safe tissue expansion. Plast Reconstr Surg 1986;77:416-20. [Crossref] [PubMed]
- Hallock GG. Puncture threshold prior to leakage from tissue expander reservoirs. Plast Reconstr Surg 1988;82:666-8. [Crossref] [PubMed]
Cite this article as: Hallock GG. Tissue expansion for improvement of the perforator flap donor site. J Xiangya Med 2018;3:11.


