
Role of conventional cytology and cell block methods for diagnosis of malignant pleural effusions
Introduction
Pleural effusion is a clinical condition characterized by excessive accumulation of fluid inside the pleural cavity. The causes that determine the formation of an effusion can be many, among them, heart failure, pneumonia, tuberculosis but also metastatic tumors or cancer of the pleural cavity. The cytological analysis of the pleural fluid is the first-line diagnostic test together with the biochemical analysis, especially in the suspicion of malignancy (1,2). In fact, thoracentesis is a simple procedure with low complication rates (3) and therefore more accessible and cost-effective than potentially higher performance methods such as video assisted thoracoscopy (VAT). The guidelines from the American College of Chest Physicians (ACCP) and the British Thoracic Society (BTS), recommend cytological smear (CS) of two samples of pleural effusions (4,5). If procedures are non-diagnostic, more invasive investigations such as image-guided pleural biopsy or thoracoscopic biopsy are recommended. However, the international literature of the past three decades describes a sensitivity range of 40–87% of pleural fluid cytology for the detection of MPE (4-7). This also varies with the histopathology of the tumor; in fact, sensitivity for adenocarcinoma was reported as 60% in a study versus only 30% for mesothelioma and MPE due to adenocarcinoma is more easily diagnosed than squamous cell carcinoma, sarcoma and lymphoma (5,8). The limits of the CS are in fact linked to poor morphological details often due to the overlapping of cells, the presence of inflammatory cells that can overlap neoplastic cells and the loss of important morphological characteristics in the sample preparation phase (9). The cell block (CB) method overcomes these limits because it allows the study of tissue architecture and a better conservation of the morphological features which allows a better differentiation between malignant and non-malignant cells, but also for the further characterization of cells with special stains and ICC (10). The CB technique is an ancient method for assessing body cavity fluids. Scientific guidelines indicate that the preparation of CB from pleural effusion samples, in addition to CS, allows the “microhistology” of the solid cell portion which can lead to greater diagnostic accuracy. Its main advantage is the preservation of the tissue architecture, obtaining multiple sections for special staining and immunocytochemistry (10-12). However, it is not used in routine daily clinical practice but performed only at the discretion of the pathologist or clinician. However, it is a simple method that requires no special training or tools. It is safe, cost-effective and reproducible even in rural areas with limited resources (13). On the contrary, in our institution all pleural fluids are used to obtain both CS (a conventional smear and a thin layer) and CB. The aim of the study is to evaluate the diagnostic accuracy, sensitivity and specificity of this combined approach, referring to histological confirmation or clinical data. We present the following article/case in accordance with the STROBE reporting checklist (available at http://dx.doi.org/10.21037/jxym-20-66).
Methods
Patients were enrolled after retrospective research using the laboratory information system. The database included cytopathological case number, gender, cytological diagnosis. Pleural effusion samples obtained between January 2015 and December 2019 were included with the aim of assessing sensitivity, specificity, negative and positive predictive value, diagnostic accuracy, negative and positive likelihood ratio of this combined approach (CS+CB) for the detection of MPE referring to the gold standard, i.e., histological confirmation, and clinical data, if histology was not available.
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and was approved by institutional ethics committee. Informed consent was taken from the patients.
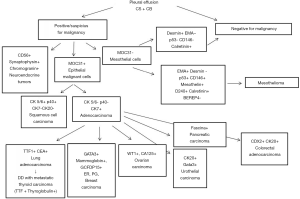
One hundred thirty-two patients were male (59.2%) and 91 were female (40.8%). Fresh PE was centrifuged at 1.750 rpm for 10 min and the supernatant removed. A few drops were drawn from the sediment and one direct slide smear was prepared and submitted for May-Grünwald-Giemsa staining. Another smear was prepared using a ThinPrep method and this slide was fixed and submitted for Papanicolaou staining. To prepare CB, Agar solution was added to the sediment, followed by refrigeration to form a solid clot. The clot was fixed in 10% neutral buffered formalin solution and automatically processed into a paraffin-embedded block. Two histological slides were cut and hematoxylin and eosin (H&E) and PAS staining were performed. All samples were reviewed by two cytopathologists. ICC stains were applied in cases of PE positive or suspicious for malignant cells as described in Figure 1. For cytological diagnosis, the conventional diagnosis criteria were divided into three categories as benign, malignant, and suspicious for malignancy. In CB examination, histopathological diagnosis was done in cases with sufficient cell counts. Immunocytochemistry panels on CB sections were applied according to the suspected tumor type based on cytomorphology.
Statistical analysis
Statistical analysis was performed using a statistical software package (SPSS 25.0; SPSS Inc., Chicago, IL, USA) for Windows. Mean ± standard deviation (SD) (normally distributed data), median and range (non-normally distributed data) and percentage frequencies was calculated. Within-patient comparisons were made by paired t-test and Fisher’s exact test, as appropriate, at significance levels of P<0.05.
Results
Out of 223 patients, 68 were positive for malignancy (30.5%), 6 (2.7%) were suspect for malignant disease, 5 (2.2%) are inadequate samples for diagnosis. The number of females with malignant cytology (37 cases, 40.7%) was significantly higher than males (31 cases, 23.5%) (P=0.0074) (Table 1). The main cause of MPE was metastatic adenocarcinoma. Using the combined approach and integrating cytomorphology with ICC, like described in Figure 1, we have identified site of origin of malignant cells in 50 cases (73.5%). With reference to histological and clinical data (for patients in whom the biopsy was not performed), the combined approach (CS+CB) on pleural effusion showed a sensitivity of 92%, a specificity of 96%, a positive predictive value of 92% and a negative predictive value of 96% with a diagnostic accuracy of 95%. The positive likelihood ratio was 23 and the negative likelihood ratio was 0.08. The most common primary site involved in the MPE for the total cohort was lung (23 patients, 33.8%), followed by breast cancer (14 cases, 20.6%), gastrointestinal cancer (7 patients, 10.3%; in detail 4 colon, 2 pancreas and biliary tract, 1 stomach), mesothelioma (6 patients, 8.8%), ovarian-carcinoma (4 patients, 5.9%), head-neck squamous carcinomas (2 patients, 2.9%), lymphoma (1 patient, 1.5%), endometrial cancer (1 patient, 1.5%) and neuroendocrine tumor (1 patient, 1.5%). For the remaining 9 patients (13.2%), the pathologists were able only to discern malignancy as adenocarcinoma in 6 cases and epithelial origin of MPE in 3 cases, but could not go further because of insufficient materials for further ancillary studies, lack of clinical history of a primary tumor site or nonspecific morphology and/or immunocytochemical (ICC) staining patterns. Stratifying by gender, the most common etiology for a malignant pleural effusion (MPE) for women was breast cancer (14 patients, 37.8%), followed by lung cancer (9 patients, 24.3%) ovarian cancer (4 patients, 10.8%) and gastrointestinal cancer (3 patients, 8.1%). For men the common etiology was lung cancer (14 patients, 4.2%) followed by gastrointestinal cancer (4 patients, 12.9%) and mesothelioma (4 patients, 12.9%). ICC studies were performed on 74 (33.2%) CB, with a median of 3 (25th to 75th percentiles 1–4) immunostains per case. From a total of 231 immunostainings, TTF-1 (35, i.e., 47.3% of ICC), CK 7 (29, i.e., 39.2% of ICC), CK 20 (24, i.e., 32.4% of ICC), MOC31 (16, i.e., 21.6% of ICC), WT1 (15, i.e., 20.3% of ICC), calretinin (12, i.e., 16.2% of ICC), mammaglobin (10, i.e., 13.5% of ICC), estrogen receptor (10, i.e., 13.5%), D240 (9, i.e. 12.2% of ICC), CDX2 (8, i.e., 10.8% of ICS), EMA (7, i.e., 9.5% of ICS), GATA3 (6, i.e. 8.10% of ICS), CK5/6 (5, i.e., 6.8% of ICC) were the most commonly used.
Table 1
| Year | PE positive for malignancy, N (%) | PE negative for malignancy, N (%) | PE suspicious for malignancy, N (%) | PE inadequate for diagnosis, N (%) | Male with MPE, N (%) | Female with MPE, N (%) | Male negative for MPE, N (%) | Female negative for MPE, N (%) |
|---|---|---|---|---|---|---|---|---|
| 2015 | 6 (2.7) | 39 (17.5) | 0 (0) | 0 (0) | 1 (0.4) | 5 (2.2) | 28 (12.6) | 11 (4.9) |
| 2016 | 22 (9.9) | 44 (19.7) | 2 (0.9) | 0 (0) | 10 (4.5) | 12 (5.4) | 25 (11.2) | 19 (8.5) |
| 2017 | 19 (8.5) | 22 (9.9) | 2 (0.9) | 4 (1.8) | 9 (4.0) | 10 (4.5) | 16 (7.2) | 6 (2.7) |
| 2018 | 12 (5.4) | 20 (9.0) | 1 (0.4) | 0 (0) | 9 (4.0) | 3 (1.3) | 12 (5.4) | 8 (3.6) |
| 2019 | 9 (4.0) | 19 (8.5) | 1 (0.4) | 1 (0.4) | 2 (0.9) | 7 (3.1) | 13 (5.8) | 6 (2.7) |
| Total | 68 (30.5) | 144 (64.6) | 6 (2.7) | 5 (2.2) | 31 (13.9) | 37 (16.6) | 94 (42.2) | 50 (22.4) |
PE, pleural effusion; MPE, malignant pleural effusion.
Discussion
Thoracentesis is a non-invasive technique and widely available in many hospitals. This technique allows to obtain the pleural fluid to perform cytological investigations in the MPE suspicion. The combined CS and CB techniques increases the diagnostic value of CS alone (14-20). CS is the first line method in association with biochemical tests if there is pleural effusion and a suspicion of malignancy. However, literature data suggest a low sensitivity of this method.
The major limitation of this method is related to the low ability to distinguish reactive mesothelial cells from neoplastic mesothelial and epithelial cells. These limits are due to the artifacts related to the preparation and staining techniques, but also to the limit of the absence of a three-dimensionality that causes overcrowding of cells and overlap with poor resolution and difficulty of interpretation. The CB technique allows to overcome many of these limitations
In fact, allowing to obtain a histological piece, it provides more morphological details such as the presence of cellular spheres, papillae, clusters, it also allows to obtain multiple sections and stainings and above all ICC stains (9,13,14,17,21).
Last but not least, the use of CB gives the possibility to conserve the material and to use it for molecular biology tests such as histological samples (11,12,14,22,23). However, despite the guidelines invite to use CB for the advantages of this well established technique (24), CB is considered time consuming and is performed only in few Institution (5,25). A recent survey among last two-year Spanish residents of Pneumology or Internal Medicine showed that only 16% of 139 responders in their clinical practice actively ordered a CB when confronted with a suspected malignant effusion, whereas the remaining either never did (27%) or left the decision to the pathologist discretion (57%) (26). In our Institute CB was carried out on all pleural fluids received for cytological investigations. In addition to the CB, 2 smears are performed, one thin layer smear and one conventional smear, then two stains are performed (Papanicolaou and MG) and a cytochemical stain with PAS. An ICC panel is also performed on samples showing cells suspected for malignancy or with atypical characteristics, which provides an average of 3 antibodies addressed as described in Figure 1. Using this protocol, the sensitivity and specificity data and the diagnostic accuracy are widely increased compared to literature data (27-29). The performance data obtained are also superior to two successive samplings with repeated thoracentesis (30), confirming the usefulness of the combined cytological diagnosis compared to more invasive and less available methods, such as pleural biopsy in VAT. In keeping with our data it should be noted that in specialized centers, mesothelioma can be diagnosed using pleural fluid cytology in up to 73% of the cases (31), highlighting the importance of the cytologist’s skills. Currently, experienced cytopathologists promote cytological diagnosis of mesothelioma without the need for biopsy, if pleural fluid cytoarchitectural and ICC features are conclusive. The diagnostic protocol for MPE, used in our center, also made possible to identify the site of origin in 73.5% of cases. The frequency of malignancy shown are in agreement with the literature (32,33), which highlights a prevalence of lung tumors in men and breast tumors in women. In our experience, the frequency of hematological tumors is lower than the literature data but this is probably linked to the presence of a flow cytometry service in another department, constituting a bias in our study. Other limitation is that it is a retrospective study and pathologists were not blinded of clinical data. Furthermore, we have analyzed all pleural fluids received at the Pathology and Histology Unit and not all those performed at our hospital; this could generate a sample selection bias. Finally, in half of pleural effusions the malignancy was not confirmed by histological data but it was based on solid clinical data.
Conclusions
In conclusion, considering the challenging implications due to the presence of malignant cells in pleural effusions, an accurate cytologic evaluation represents a critical and mainstream diagnostic tool being easy, safe and cost-effectiveness, reducing complications of a more aggressive biopsy procedure. Our study confirms that the combined approach (CB+CS) increases the diagnostic yield in MPE. CB method was a simple, inexpensive and did not require any special training or instrument. Bridging the gap between cytology and histology CB should be considered as a useful adjuvant technique along with CS in evaluating pleural fluid cytology, being the preferred substrate for ICC. Our study suggests that pathologists should be use this approach in their clinical practice and clinicians should be encouraged to request CB, along with the conventional routine cytology, in order to increase diagnostics sensitivity and establish a more definitive cytopathological diagnosis.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Journal of Xiangya Medicine, for the series “Malignant Pleural Effusion”. The article has undergone external peer review.
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/jxym-20-66
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jxym-20-66). The series “Malignant Pleural Effusion” was commissioned by the editorial office without any funding or sponsorship. RC and DD served as the unpaid Guest Editors of the series. DD serves as an unpaid editorial board member of Journal of Xiangya Medicine from Nov 2019 to Oct 2021. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and was approved by institutional ethics committee (No. PA 12 01, CHT 03-2019). Informed consent was taken from the patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Porcel JM. Diagnosis and characterization of malignant effusions through pleural fluid cytological examination. Curr Opin Pulm Med 2019;25:362-8. [Crossref] [PubMed]
- Light RW, Macgregor MI, Luchsinger PC, et al. Pleural effusions: the diagnostic separation of transudates and exudates. Ann Intern Med 1972;77:507-13. [Crossref] [PubMed]
- Gaur DS, Chauhan N, Kusum A, et al. Pleural fluid analysis - Role in diagnosing pleural malignancy. J Cytol 2007;24:183-8. [Crossref]
- Rivera MP, Mehta AC, Wahidi MM. Establishing. The diagnosis of lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:e142S‐e165S.
- Hooper C, Lee YC, Maskell N, et al. Investigation of a unilateral pleural effusion in adults: British Thoracic Society Pleural Disease Guideline 2010. Thorax 2010;65:ii4-ii17. [Crossref] [PubMed]
- McGrath EE, Anderson PB. Diagnosis of pleural effusion: a systematic approach. Am J Crit Care 2011;20:119-27; quiz 128. [Crossref] [PubMed]
- Gupta S, Sodhani P, Jain S. Cytomorphological profile of neoplastic effusions: an audit of 10 years with emphasis on uncommonly encountered malignancies. J Cancer Res Ther 2012;8:602-9. [Crossref] [PubMed]
- Bielsa S, Panadés MJ, Egido R, et al. Accuracy of pleural fluid cytology in malignant effusions. An Med Interna 2008;25:173-7. [PubMed]
- Köksal D, Demırağ F, Bayız H, et al. The cell block method increases the diagnostic yield in exudative pleural effusions accompanying lung cancer. Turk Patoloji Derg 2013;29:165-70. [Crossref] [PubMed]
- Jing X, Li QK, Bedrossian U, et al. Morphologic and immunocytochemical performances of effusion cell blocks prepared using 3 different methods. Am J Clin Pathol 2013;139:177-82. [Crossref] [PubMed]
- Nathan NA, Narayan E, Smith MM, et al. Cell block cytology. Improved preparation and its efficacy in diagnostic cytology. Am J Clin Pathol 2000;114:599-606. [Crossref] [PubMed]
- Kuhlmann L, Berghäuser KH, Schäffer R. Distinction of mesothelioma from carcinoma in pleural effusions. An immunocytochemical study on routinely processed cytoblock preparations. Pathol Res Pract 1991;187:467-71. [Crossref] [PubMed]
- Kim JH, Kim GE, Choi YD, et al. Immunocytochemical panel for distinguishing between adenocarcinomas and reactive mesothelial cells in effusion cell blocks. Diagn Cytopathol 2009;37:258-61. [Crossref] [PubMed]
- Shivakumarswamy U, Arakeri SU, Karigowdar MH, et al. Diagnostic utility of the cell block method versus the conventional smear study in pleural fluid cytology. J Cytol 2012;29:11-5. [Crossref] [PubMed]
- Ghosh I, Dey SK, Das A, et al. Cell block cytology in pleural effusion. J Indian Med Assoc 2012;110:390-2, 396. [PubMed]
- Assawasaksakul T, Boonsarngsuk V, Incharoen P. A comparative study of conventional cytology and cell block method in the diagnosis of pleural effusion. J Thorac Dis 2017;9:3161-7. [Crossref] [PubMed]
- Bhanvadia VM, Santwani PM, Vachhani JH. Analysis of diagnostic value of cytological smear method versus cell block method in body fluid cytology: study of 150 cases. Ethiop J Health Sci 2014;24:125-31. [Crossref] [PubMed]
- Grandhi B, Shanthi V, Rao MN, et al. The diagnostic utility of cell block as an adjunct to cytological smears. Int J Med Res Health Sci 2014;3:278-84. [Crossref]
- Porcel JM, Quirós M, Gatius S, et al. Examination of cytological smears and cell blocks of pleural fluid: Complementary diagnostic value for malignant effusions. Rev Clin Esp 2017;217:144-8. [Crossref] [PubMed]
- Thapar M, Mishra RK, Sharma A, et al. Critical analysis of cell block versus smear examination in effusions. J Cytol 2009;26:60-4. [Crossref] [PubMed]
- Ugurluoglu C, Kurtipek E, Unlu Y, et al. Importance of the cell block technique in diagnosing patients with non-small cell carcinoma accompanied by pleural effusion. Asian Pac J Cancer Prev 2015;16:3057-60. [Crossref] [PubMed]
- DeMaio A, Clarke JM, Dash R, et al. Yield of Malignant Pleural Effusion for Detection of Oncogenic Driver Mutations in Lung Adenocarcinoma. J Bronchology Interv Pulmonol 2019;26:96-101. [Crossref] [PubMed]
- Roy-Chowdhuri S. Advances in Molecular Testing Techniques in Cytologic Specimens. Surg Pathol Clin 2018;11:669-77. [Crossref] [PubMed]
- Dekker A, Bupp PA. Cytology of serous effusions. An investigation into the usefulness of cell blocks versus smears. Am J Clin Pathol 1978;70:855-60. [Crossref] [PubMed]
- Jonasson JG, Ducatman BS, Wang HH. The cell block for body cavity fluids: do the results justify the cost? Mod Pathol 1990;3:667-70. [PubMed]
- Porcel JM, Cases-Viedma E, Bielsa S. A survey to medical residents on the performance of diagnostic and therapeutic thoracenteses: a training gap? Rev Clin Esp 2016;216:474-80. [Crossref] [PubMed]
- Rodríguez-Panadero F. Medical thoracoscopy. Respiration 2008;76:363-72. [Crossref] [PubMed]
- Porcel JM, Esquerda A, Vives M, et al. Etiology of pleural effusions: analysis of more than 3,000 consecutive thoracenteses. Arch Bronconeumol 2014;50:161-5. [Crossref] [PubMed]
- Arnold DT, De Fonseka D, Perry S, et al. Investigating unilateral pleural effusions: the role of cytology. Eur Respir J 2018;52:1801254. [Crossref] [PubMed]
- Garcia LW, Ducatman BS, Wang HH. The value of multiple fluid specimens in the cytological diagnosis of malignancy. Mod Pathol 1994;7:665-8. [PubMed]
- Segal A, Sterrett GF, Frost FA, et al. A diagnosis of malignant pleural mesothelioma can be made by effusion cytology: results of a 20 year audit. Pathology 2013;45:44-8. [Crossref] [PubMed]
- Lew M, Cantley R, Heider A, et al. Diagnosis and categorization of malignant effusions: A 6-year review from a single academic institution. Diagn Cytopathol 2020; [Epub ahead of print]. [Crossref] [PubMed]
- Güldaval F, Anar C, Polat G, et al. Contribution of Cell Block Obtained by Thoracentesis in the Diagnosis of Malignant Pleural Effusion. J Cytol 2019;36:205-8. [Crossref] [PubMed]
Cite this article as: Chiatamone Ranieri S, Di Leonardo G, Coletti G, Dal Mas A, Brancone ML, Crisci R, Divisi D. Role of conventional cytology and cell block methods for diagnosis of malignant pleural effusions. J Xiangya Med 2020;5:36.


