
An unusual concurrent dermatological and neurological manifestation in an elderly woman with eosinophilic granulomatosis with polyangiitis: a case report and literature review
Introduction
Eosinophilic granulomatosis with polyangiitis (EGPA) strikes various organ systems. This class of vasculitis attacks, principally small and medium vessels. Only 40% of EGPA cases test positive for anti-neutrophil cytoplasmic antibodies (ANCAs), with cardiac involvement contributing to the likelihood of ANCA negativity in EGPA (1-3).
EGPA is considerably uncommon, still conceivably life-threatening vasculitis (4). Augmented therapeutic approaches for EGPA and other vasculitides have modified acute presentation into chronic ones. Hence EGPA is accountable for marked morbidity and a consequence on the employability and quality of life (5).
Notwithstanding eosinophilia (pulmonary and peripheral) with infiltrates on chest X-ray features. Eosinophilic vasculitis appears in the background of asthma. The preoccupation of various end-organs remains the central hallmarks that discriminate EGPA from other pulmonic eosinophilia complexes. The recent heightened frequency recorded, especially in connection to asthma therapeutics (6).
The estimated EGPA incidence is 1 to 3 per million annually, commonly in females. Further, it can be diagnosed at any age but usually between 40–60 years with a mean onset at 48 years and infancy being an exception (7).
Moreover, neuropathy manifests in 65% to 80% of EGPA cases, and nearly 30% of the victims lack another organ involvement (8). Dermatological signs occur in 40% to 81% of EGPA patients, while 14% manifest as initial symptoms (9).
The pathogenesis and purposes of ANCA in EGPA principally concealed (10). The EGPA linked to multifactorial medications or allergens’ vulnerability triggers. The genetic setting, especially the relationship with HLA-DRB4. Although evidence is limited, eosinophils appear to have a significant role in pathogenesis in all three EGPA clinical steps. Eosinophils activation provokes tissue destruction by delivering granule proteins. Hence, eosinophil aggregation and activation clinically exhibit allergic inflammation, thrombosis, and fibrosis. Up-regulation of interleukins, especially IL-5, IL-4, and IL-13, stated. Similarly, prominent IgG4 and IgE responses (humoral immunity) dysregulation reported (11,12).
The mechanism of hypercoagulability is not wholly apparent; proposed factors include the initiation of the clotting cascade. The restraint of endothelial thrombomodulin vascularly plays the same role. Meanwhile, Eosinophils can directly damage a nerve, yet, ischemic nerve insult due to eosinophilic infiltration of vascular walls is a principal mechanism in EGPA mono neuritis multiplex (12).
Compared to other ANCA-associated vasculitis patterns, EGPA rareness and unique hallmarks have slowed the advancement of studies for many years (10). In up to 1/3 of EGPA cases, likely to confront diagnostic obstacles for >6 months hence a meticulous (comprehensive history/examination) and systematic strategy are compelled to execute the diagnosis (13). Enhanced EGPA recognition is needed to assist in the appropriate diagnosis and treatment (4).
The management of EGPA nevertheless has many shortages, with various drugs under investigation. IL-5 plays a crucial part in the increase and maturation of eosinophil progenitors, emigration, and prime eosinophils’ endurance. Several pharmacological choices target the modification of IL-5 titers. EGPA punctually responds to glucocorticoids, although combinations with immunosuppressants documented in most cases. Anti-CD20 pharmacological choices confirmed encouraging outcomes in pilot researches. Around 50% of untreated victims die in three months of diagnosis. Comparatively, >70% treated cases have a six-year survival (6,11,12,14).
Here, we report an 81-year-old female case with severe EGPA 10 years post-Asthma with central and peripheral neurological manifestation. The presentation accompanied by other systemic manifestations in cardiac, gastrointestinal, hematological, and pulmonary systems presented initially with cutaneous manifestations. Further, we review other EGPA cases in which cutaneous manifestations co-occurred with the neurological symptoms/signs to establish the likelihood of ANCA in cor-morbidity. We present the following article in accordance with the CARE reporting checklist (available at http://dx.doi.org/10.21037/jxym-20-80).
Case presentation
An 81-year-old female presented in the Neurology department with a 5 days history of left limb weakness, which was more marked at the peripheral and impaired walking, accompanied by numbness of both feet. She also reported a history of acute onset abdominal pain and lack of appetite but a healthy stool and urine frequency and color. There was no history of nausea, vomiting, headache, fever, and joint pain. Further, no history of cough or chest tightness, exertional dyspnea, or paroxysmal nocturnal dyspnea reported.
She reported being known hypertensive on nifedipine tabs. She had a history of cataract surgery but denied diabetes history and coronary heart diseases. Ten years prior, diagnosed with tuberculosis and asthma. Moreover, two weeks before the admission, she presented to the dermatology department of our hospital with rashes in which physical examination revealed palpable purpuric purple rashes localized on the bilateral lower limbs and thought to be Henoch-Schönlein purpura (HSP). Hence, initiated on high dose corticosteroids, she denied the history of drug or food allergy.
General examination revealed unaltered conscious level, orientation, and cooperation but slight edema of both feet, a diffuse palpable purpuric rash on the bilateral lower extremities with the blood pressure 120/80 mmHg, Temperature 36 °C, pulse rate 88 bpm, respiratory rate 18 times/min. The neurological examination revealed intact cranial nerves, normal muscle tone in all limbs. The muscle strength was adequate except for the left lower limb, which was grade 4/5 with normal bilateral the tendon reflexes both his lower and upper extremities with symmetrical sensation and intact cerebellar tests. Other systems produced unremarkable results.
On the first visit as an outpatient blood examination (Table 1) showed leukocytosis of 15.9×109/L, elevated eosinophilia count of 9.46×109/L (59.5%), RBC 3.12×1012/L and hemoglobin level of 96.7 g/L. Two weeks later, after admission to the neurology department, laboratory investigation demonstrated a leukocyte count of 7×109/L with a neutrophil count of 25%, lymphocyte count 16%, and high eosinophil percentage of 55.00%, while hemoglobin level decreased to 88.90 g/L with erythrocyte count of 3.00×1012/L and hematocrit of 27%, a platelet count of 270×109/L, N 26.4%, L 10.5%. Urinalysis showed leukocytosis of 3 WBCs/HPF but negative for protein. Likewise, no occult blood, and heavy metal intoxication observed. Meanwhile, stool analysis tested positive for an occult blood test and negative for ova or parasites. Cytoplasmic antineutrophilic cytoplasmic antibodies (cANCAs) and perinuclear ANCAs (pANCAs) were negative. No abnormality recorded from Anti-double-stranded DNA antibodies and anticardiolipin antibodies.
Table 1
| Investigations | Reference range | Value |
|---|---|---|
| Total white cell count (×109/L) | 3.50–9.50 | 15.9 |
| Eosinophil count (×109/L) | 0.4–0.8 | 9.46 |
| Haemoglobin levels (g/L) | 115.00–150.00 | 88 |
| Erythrocyte count (×109/L) | 3.80–8.10 | 3.00 |
| ANCA | – | Negative |
| Stool analysis | – | Positive for occult blood |
| Echocardiography | – | Aortic regurgitation |
ANCA, antineutrophilic cytoplasmic antibodies.
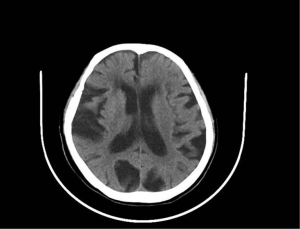
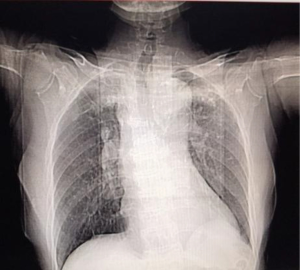
Serum electrolytes, thyroid, liver, and renal function tests had no abnormalities. The initial computed tomography (CT) of the head (Figure 1) after admission revealed no cerebral hemorrhage or space-occupying lesion signs. However, bilateral paraventricular, bilateral basal ganglia lacunar cerebral infarction, cerebral atrophy, and nasosinusitis noted. Chest CT (Figure 2) showed multiple fibrous and sclerotic foci in both lobes (upper and lower) of both lungs and atherosclerosis of the aorta and coronary artery. The total cholesterol was 3.75 mmol/L, low-density lipoprotein cholesterol LDL-C was 2.03 mmol/L, α-Hydroxybutyrate Dehydrogenase 256 µ/L, myoglobin 134.73 ng/mL, Lactic acid dehydrogenase (LDH) 304 µ/L, BNP 74 pg/mL, and high-density lipoprotein cholesterol HDL-C 0.73 mmol/L. Transcranial Doppler ultrasonography (TCD) demonstrated calcified bilateral common carotid arteries. Plaque formation and bilateral middle cerebral artery insufficiency were significant.
Echocardiography revealed a regurgitant aortic valve. Ejection fraction of normal range, ECG sinus rhythm with low-voltage limb conduction noted. The color ultrasound examination reported no obvious abnormality in the liver (portal vein), gallbladder, pancreas, spleen, kidney, ureter, and bladder.
The nerve conduction studies (Table 2) showed the amplitude of the motor wave of the left upper limb median nerve reduced, the velocity slowed down, and the amplitude of the sensory conduction decreased, while in the right upper extremity amplitude of sensory conduction decreased. The ulnar nerve motor conduction of both upper limbs was blocked, and the amplitude of sensory conduction decreased. The amplitude of tibial nerve conduction decreased in both legs, yet the fibular nerve conduction did not lead to a positive waveform. The amplitude and velocity of sensory conduction of the peroneal nerve in lower limbs reduced, and there was no positive wave produced. The F wave was normal in the ulnar nerve in both upper limbs, the F wave frequency of the tibial nerve in left lower extremity declined while in the right lower extremity did not lead to a positive wave. This registered peripheral nerve damage of extremities involving (motor, sensory fiber, distal, and proximal nerve roots).
Table 2
| Nerve | Latency (ms) | Amplitude (ìV or mV) | Cv (m/s) | |||||
|---|---|---|---|---|---|---|---|---|
| Right | Left | Right | Left | Right | Left | |||
| Motor recordings | ||||||||
| Ulnar | 2.21 | 2.54 | 10.2 | 5.7 | 51.6 | 48.4 | ||
| Median | 3.55 | 3.71 | 6.6 | 0.21 | 54.6 | 43.1 | ||
| Tibial | 4.10 | 3.87 | 0.61 | 0.74 | Absent | Absent | ||
| Common peroneal | Absent | Absent | Absent | Absent | Absent | Absent | ||
| Sensory recordings | ||||||||
| Superficial peroneal | Absent | Absent | Absent | Absent | Absent | Absent | ||
| Sural | 3.03 | 3.21 | 2.7 | 2.4 | 33.0 | 31.2 | ||
| Ulnar | 1.98 | 2.04 | 9.2 | 6.4 | 55.6 | 53.9 | ||
| Median | 2.85 | 2.73 | 12.0 | 6.6 | 45.6 | 47.6 | ||
Cv are conduction velocity, sensory amplitudes are ìV, motor amplitudes mV.
In the picture of the patient's muscle weakness, methylcobalamin, rat nerve growth factor, and thiamine for nerve nourishment started, rehabilitation treatment followed. Moreover, antiplatelet (Clopidogrel 75 mg), lipid regulating drug (atorvastatin), was also initiated. By considering the elderly age, the aortic valve reflux not associated with any cardiac symptoms such as chest tightness, chest pain, dyspnea, and abnormal BNP values. Nevertheless, the ejection fraction was in the normal range; the patient advised to have regular check the cardiac color Doppler ultrasound and regularly record the 24-hour urine volume with the cardiac clinic follow-up.
In the course of the management, the patient’s difficulty of walking improved, skin rash subsided, and muscle strength of bilateral upper/lower limbs improved. She was discharged on the 7th day and continued with outpatient follow-up. During the follow-up phase no new symptom reported.
Discussion
EGPA is an uncommon autoimmune vasculitis of unfamiliar etiology that affects blood vessels, which are small and medium in size. Its encounter linked with Asthma. Typically affects pulmonary vessels, but due to raised blood and tissue eosinophilia, vasculitis may end up with the involvement of multiple organs (15). However, the pulmonary manifestation of EGPA often precedes the pathology’s systemic involvement by a prolonged duration (16).
In order to achieve the sensitivity of 85% and a specificity of 99% to meet the classification of EGPA, 4 of the following six features must be present as the American College of Rheumatology (ACR) recommendation: asthma manifestation, peripheral blood eosinophilia (exceeding 10%), paranasal sinus irregularity, mononeuropathy/ polyneuropathy, non-fixed pulmonary infiltrates and elevated eosinophils extravascularly (17). Our patient met 4 of the 6 ACR criteria: she had a history of Asthma, elevated eosinophilia count of 9.46×109/L (59.5%), polyneuropathy, and head CT revealed nasosinusitis. Chest CT showed multiple fibrous and sclerotic foci.
Considering cutaneous, central and peripheral, nervous system, cardiac, hypertension, gastrointestinal, hematological, and pulmonary manifestation. The differential diagnoses of EGPA vasculitis include HSP, Wegener’s granulomatosis (WG) and microscopic polyangiitis (MPA) (7). Based on the grounds of purpuric rashes with lower limb predominance, abdominal pain, and occult stool blood findings in our patient, HSP was considered an important differential diagnosis. Nevertheless, unlike EGPA, HSP lacks three unique clinical aspects, which are characteristic of the EGPA. The prodromal stage in which allergic rhinitis and asthma are the hallmarks may persevere for several years. Peripheral eosinophilia and eosinophilic tissue infiltration stage. Systemic vasculitis with multiple organ involvement stages marking the terminating stage (18). Additionally, prior asthma presentation and the presence of eosinophilia in our patient ruled out MPA and WG (19).
Neurological signs in EGPA are principally neuropathy, commonly presenting as polyneuropathy, mono neuritis multiplex, or cranial nerve involvement (20). In this case was peripheral polyradiculoneuropathy marked with unilateral peripheral left limb weakness, numbness, and impaired walking was the presentation confirmed with the EMG. The contemplated pathogenesis points to infiltration and degranulation of eosinophils, cationic proteins, and essential proteins. Causing cytotoxicity and damaged endothelial cells, leading to multi-organ vasculitis (15).
The central nervous system (CNS) entanglement is comparatively unusual, with most cases associated with ischaemic infarctions due to fibrinoid necrosis and inflammation of CNS blood vessels. Likewise, cardiac embolism, aortic wall inclusively, is one of the likely pathogenesis of cerebral infarction in EGPA (21,22). Our patient presented with both acute cerebral infarctions evidenced by Figure 1 head CT showing bilateral paraventricular, lacunar infarction of the basal ganglia. Moreover, peripheral polyradiculoneuropathy evidenced by (Table 2). Moreover, CT confirmed the presence of atherosclerosis of the aorta and coronary artery. Two cases were similar to our previous patient reported in 1995 and 2007 with dermatological manifestation, peripheral nervous system (PNS), and CNS involvement. However, unlike our case, the CNS manifestation was oculomotor nerve palsy and ischemic optic neuropathy, respectively. We have instead reported bilateral paraventricular, basal ganglia lacunar infarction (23,24). Moreover, other remaining previous cases (Table 3) with cutaneous and nervous system manifestation reported involving either CNS or PNS but not both systems, unlike this patient. 33~53=25~49,
Table 3
| Ref. | Author/year | Age (years)/sex | Neurological manifestation | Skin | ANCA | Eosinophils | Other manifestation |
|---|---|---|---|---|---|---|---|
| (25) | Dicken, 1978 | 53/M | Yes | Yes | Not reported | 9,300 cells/mm3 | Asthma |
| (26) | Finan, 1984 | 53/F | Yes | Yes | Not reported | 1,260 cells/mm3 | Asthma, ENT, RM |
| 47/F | Yes | Yes | Not reported | 3,000 cells/mm3 | Asthma, ENT, PLM | ||
| 27/M | Yes | Yes | Not reported | 8,770 cells/mm3 | Asthma, PLM, RN | ||
| 42/M | Yes | Yes | Not reported | 1,250 cells/mm3 | Asthma, RM, RN | ||
| 52/M | Yes | Yes | Not reported | 1,700 cells/mm3 | Asthma, PLM | ||
| (27) | Murray,1989 | 49/M | PN | Yes | Not reported | 52% | Asthma, RM |
| (28) | Chen, 1992 | 38/M | PN | Yes | NEG | 47% | NO Asthma, PLM |
| (23) | Abe-Matsuura, 1995 | 34/M | CN 3 palsy, PN | Yes | Not reported | 69% | Asthma, HTN, CD |
| 41/M | PN | Yes | NEG | 57% | Asthma | ||
| (29) | Stübiger, 1999 | 53/F | Amaurosis fugax | Yes | NEG | 20% | Asthma |
| (30) | Watson, 2004 | 32/F | Rhabdomyolysis | Yes | POS | 4,690 cells/mm3 | Asthma, PLM, RN |
| (31) | Wolf, 2005 | 42/M | PN | Yes | POS | 5,000 cells/mm3 | Asthma, PLM, RN, GI |
| (32) | Kawakami, 2006 | 42/M | PN | Yes | NEG | 19.4% | Asthma |
| (33) | Shimauchi, 2007 | 53/F | PN | Yes | NEG | 7,200 cells/mm3 | Asthma, GI |
| (24) | Kawasaki, 2007 | 71/F | Ischemic optic neuropathy, PN | Yes | Not reported | 55% | Asthma |
| (34) | Skrapari, 2008 | 50/F | Visual loss, PN | Yes | NEG | 20% | Asthma, PLM |
| (35) | Uehara, 2009 | 68/F | PN | Yes | NEG | 9,160 cells/mm3 | Asthma, PLM |
| (36) | Lestre, 2009 | 47/F | PN | Yes | NEG | 60% | Asthma, PLM |
| (37) | Vaglio, 2009 | 51/M | PN | Yes | POS | 52% | Asthma |
| (38) | Ishibashi, 2011 | 50/F | PN | Yes | NEG | 46% | Asthma |
| (39) | Gulati, 2012 | 61/M | Basal ganglion infarction | Yes | NEG | Not reported | Asthma, GI |
| (15) | Xiang, 2012 | 56/F | PN | Yes | POS | 1.5% | Asthma |
| (40) | Kumano, 2012 | 54/F | PN | Yes | NEG | 17.6% | Asthma, PLM, CD |
| (41) | Tanaka, 2012 | 77/F | Ischemic stroke | Yes | NEG | 52.1% | Asthma, CD |
| (42) | Cheng, 2012 | 60/M | Cerebral and cerebellar infarcts | Yes | POS | 64% | Asthma, ENT |
| (1) | Isawa, 2013 | 55/F | PN | Yes | NEG | 15,645 cells/mm3 | Asthma, GI, CD |
| (43) | Sulaiman,2014 | 50/M | PN | Yes | NEG | 33% | Asthma, CD |
| (44) | Kawakami, 2016 | 59/M | PN | Yes | NEG | 73% | Asthma |
| (45) | Marques, 2017 | 52/F | PN | Yes | POS | 4,320 cells/mm3 | Asthma, RN, CD |
| (46) | Kuftinec, 2017 | 28/F | PN | Yes | Not reported | 56% | Asthma, GI |
| Current case, 2020 | 81/F | Paraventricular, basal ganglia lacunar infarction, cerebral atrophy, PN | Yes | NEG | 59.5% | Asthma, GI, HTN, CD, PLM |
EGPA cases presented with Neurological and Dermatological manifestation literature review (PubMed search from 1 January 1958 to January 2020). Neurological manifestation marked by “Yes” was not specified in the literature. ANCA, anti-neutrophil cytoplasmic antibodies; NEG, negative; POS, positive; PN, peripheral neuropathy; ENT, ear, nose, throat; RM, rheumatological; PLM, pulmonary; RN, renal; CD, cardiac; HTN, hypertension; GI, gastrointestinal.
The mechanism of cardiac involvement in EGPA connected to eosinophil degranulation, which prompts inflammatory changes (47). Further, EGPA valvular heart disease correlates with endomyocardial fibrosis affecting the papillary muscles and not valvular infiltration directly (48). Moreover, the novel researches have confirmed the high likely-hood of ANCA-negative EGPA victims to present with cardiac involvement than ANCA-positive cases. Similarly, our patient exhibited aortic valve regurgitation and a negative ANCA test (1). In 2012 comparable cases with the history of Asthma, purpuric rash, nervous system manifestation, and valvular heart disease published. However, unlike in our case, PNS involvement was not a demonstration. Nevertheless, the valvular heart disease was mitral valve regurgitation rather than aortic valve regurgitation, as in our patient (41).
Notwithstanding the Five-Factor Score (FFS) immunosuppressants adjunct to glucocorticoids recommendation in victims with FFS >1 (49,50). Based on the collection of clinical trial outcomes, there is neither approved therapeutics for EGPA nor fixed recommendations (10). Nevertheless, the FFF formulated to estimate disease prognosis, but the debate on accommodating it to the therapeutic regimen remains discussed, with general guidance still contradictory. The therapy approach should accommodate each EGPA patient’s symptoms, including severity, organ entanglement, comorbidities prognosis, and age (51,52). Further, Tanaka and colleagues in 2012, reported a comparable case to ours managed with oral corticosteroid treatment with a good consequence (41). Therefore, taking into account the clinical evidence and judgment based on the age and other risk factors in our patient, management involved tapered corticosteroids; never the less CNS, PNS, cardiac was addressed accordingly. Yet, GI and dermatological symptoms resolved earlier.
The peripheral nerves and dermatological quandary are the two most common EGPA displays (3,49). Comarmond and colleagues, in 2013, observed peripheral neuropathies in approximately 51% and skin lesions in approximately 40%, while patients tested positive for ANCA-positive were 31.0%. Likewise, Sinico and colleagues in 2005 in a multicenter study found that up to 84% of the cases with peripheral neuropathy had ANCA-positive while and ANCA-negative was more frequently in cases with cardiac exhibitions (2,53). However, in these two studies, patient classification criteria based on the 1990 ACR criteria; 4 of the six features needed to meet the classification, it is not clear if the nervous system and dermatological co-occurred in those cases. Hence it is no distinct weather the neurological manifestation reported co-occurred with dermatological manifestation.
In that context, we reviewed other cases with neuro-dermatological co-morbidity (Table 3). We found: (I) this patient is the oldest patient to be reported with co-occurrence of nervous system and the dermatological manifestation of EGPA; (II) and the first patient to present with both lacunar infarction (basal ganglia) and PNS findings; (III) amongst the cases exhibited neurological, dermatological, and cardiac manifestation concurrently this is the first patient to be reported with aortic valve regurgitation. Through literature review amidst the patients’ conferred both neurological and dermatological manifestation with ANCA reports published, the results were negative in (16/22) 73% of the patients. We observed that >95% of EGPA cases with nervous and cutaneous manifestation are likely to present with Asthma or the history of Asthma.
Conclusions
EGPA is a rare disorder with unique features; hence, there is a lag in the research advancement for several years. In cases displaying the cutaneous manifestations as the initial sign of EGPA accompanying neurological signs, obtaining asthma history is mandatory as 95% of EGPA cases with nervous and cutaneous manifestation are likely to present with Asthma or the history of Asthma. We presented the oldest patient with co-occurrence of the neurological and the skin manifestation of EGPA. The unique presentation of both basal ganglia lacunar infarction and PNS findings reported. The cardiac manifestation of aortic valve regurgitation rather than mitral valve regurgitation observed. Reasonably, future extensive studies should explore the further prevalence of ANCA negative results when neurological manifestation co-occur with dermatological manifestation.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at http://dx.doi.org/10.21037/jxym-20-80
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jxym-20-80). The authors have no conflicts of interest to declare.
Ethical Statement:
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Isawa Y, Osada S, Omi T, et al. Eosinophilic granulomatosis with polyangiitis (Churg-Strauss Syndrome) with microscopic eosinophilic thromboembolism and cardiac involvement: report of two cases. Eur J Dermatol 2013;23:677-80. [Crossref] [PubMed]
- Sinico RA, Di Toma L, Maggiore U, et al. Prevalence and clinical significance of antineutrophil cytoplasmic antibodies in Churg-Strauss syndrome. Arthritis Rheum 2005;52:2926-35. [Crossref] [PubMed]
- Mouthon L, Dunogue B, Guillevin L. Diagnosis and classification of eosinophilic granulomatosis with polyangiitis (formerly named Churg-Strauss syndrome). J Autoimmun 2014;48-49:99-103. [Crossref] [PubMed]
- Eleftheriou D, Gale H, Pilkington C, et al. Eosinophilic granulomatosis with polyangiitis in childhood: retrospective experience from a tertiary referral centre in the UK. Rheumatology (Oxford) 2016;55:1263-72. [Crossref] [PubMed]
- Benarous L, Terrier B, Laborde-Casterot HFrench Vasculitis Study Group (FVSG), et al. Employment, work disability and quality of life in patients with ANCA-associated vasculitides. The EXPOVAS study. Clin Exp Rheumatol 2017;35:40-46. [PubMed]
- Akuthota P, Wechsler EM. Hypersensitivity Pneumonitis and Pulmonary Infiltrates with Eosinophilia. In: Harrison's principles of internal medicine. New York, NY: McGraw-Hill Education, 2018:1970-76.
- Langford CA, Fauci AS. The Vasculitis Syndromes. In: Harrison’s Principles of Internal Medicine. New York, NY: McGraw-Hill, 2018:2574-89.
- Gorelick P, Testai F, Hankey G, et al. Hankey's Clinical Neurology. London: CRC Press, 2014.
- Bosco L, Peroni A, Schena D, et al. Cutaneous manifestations of Churg-Strauss syndrome: report of two cases and review of the literature. Clin Rheumatol 2011;30:573-80. [Crossref] [PubMed]
- Furuta S, Iwamoto T, Nakajima H. Update on eosinophilic granulomatosis with polyangiitis. Allergol Int 2019;68:430-6. [Crossref] [PubMed]
- Vaglio A, Buzio C, Zwerina J. Eosinophilic granulomatosis with polyangiitis (Churg-Strauss): state of the art. Allergy 2013;68:261-73. [Crossref] [PubMed]
- Khoury P, Grayson PC, Klion AD. Eosinophils in vasculitis: characteristics and roles in pathogenesis. Nat Rev Rheumatol 2014;10:474-83. [Crossref] [PubMed]
- Yates M, Watts R. ANCA-associated vasculitis. Clin Med (Lond) 2017;17:60-4. [Crossref] [PubMed]
- Pagnoux C, Groh M. Optimal therapy and prospects for new medicines in eosinophilic granulomatosis with polyangiitis (Churg-Strauss syndrome). Expert Rev Clin Immunol 2016;12:1059-67. [Crossref] [PubMed]
- Xiang JY, Zhao YW, Fu JL, et al. Clinicopathological features of Churg-Strauss syndrome with severe nerve degeneration: a case report. Medicina (Kaunas) 2012;48:244-8. [Crossref] [PubMed]
- Lewis JB, Neilson EG. Glomerular Diseases. In: Harrison’s Principles of Internal Medicine. New York, NY: McGraw-Hill, 2018:2132-50.
- Masi AT, Hunder GG, Lie JT, et al. The American College of Rheumatology 1990 criteria for the classification of Churg-Strauss syndrome (allergic granulomatosis and angiitis). Arthritis Rheum 1990;33:1094-100. [Crossref] [PubMed]
- Lanham JG, Elkon KB, Pusey CD, et al. Systemic vasculitis with asthma and eosinophilia: a clinical approach to the Churg-Strauss syndrome. Medicine (Baltimore) 1984;63:65-81. [Crossref] [PubMed]
- Jennette JC, Thomas DB, Falk RJ. Microscopic polyangiitis (microscopic polyarteritis). Semin Diagn Pathol 2001;18:3-13. [PubMed]
- Hamati AI. Neurological Complications of Systemic Disease. In: Bradley Neurology in Clinical Practice. Philadelphia, PA: Elsevier/Saunders, 2016:205-16.
- Ullah Z, Abideen ZU, Shoaib RF, et al. Eosinophilic granulomatosis with polyangiitis presenting with multiple intracerebral haemorrhages - A case report. J Pak Med Assoc 2016;66:1481-3. [PubMed]
- Okada H. Multiple Thromboembolic Cerebral Infarctions from the Aorta in a Patient with Churg-Strauss Syndrome. J Stroke Cerebrovasc Dis 2017;26:e32-3. [Crossref] [PubMed]
- Abe-Matsuura Y, Fujimoto W, Arata J. Allergic granulomatosis (Churg-Strauss) associated with cutaneous manifestations: report of two cases. J Dermatol 1995;22:46-51. [Crossref] [PubMed]
- Kawasaki I, Shimada Y, Mori K, et al. Diagnostic and theraputic challenges. Retina 2007;27:253-8. [Crossref] [PubMed]
- Dicken CH, Winkelmann RK. The Churg-Strauss granuloma: cutaneous, necrotizing, palisading granuloma in vasculitis syndromes. Arch Pathol Lab Med 1978;102:576-80. [PubMed]
- Finan MC. Rheumatoid papule, cutaneous extravascular necrotizing granuloma, and Churg-Strauss granuloma: are they the same entity? J Am Acad Dermatol 1990;22:142-3. [Crossref] [PubMed]
- Murray NB, Barber FA, Murray KM. Asthma, eosinophilia and palpable purpura. Chest 1989;96:416-8. [Crossref] [PubMed]
- Chen KR, Ohata Y, Sakurai M, et al. Churg-Strauss syndrome: report of a case without preexisting asthma. J Dermatol 1992;19:40-7. [Crossref] [PubMed]
- Stübiger N, Schlote T, Kötter I, et al. Churg-Strauss-Syndrom. Klin Monbl Augenheilkd 1999;214:171-4. [Crossref] [PubMed]
- Watson KM, Salisbury JR, Creamer D. Purpura fulminans -- a novel presentation of Churg Strauss syndrome. Clin Exp Dermatol 2004;29:390-2. [Crossref] [PubMed]
- Wolf M, Rose H, Smith RN. Case records of the Massachusetts General Hospital. Case 28-2005. A 42-year-old man with weight loss, weakness, and a rash. N Engl J Med 2005;353:1148-57. [Crossref] [PubMed]
- Kawakami T, Soma Y, Hosaka E, et al. Churg-Strauss syndrome with cutaneous and neurological manifestations preceding asthma. Acta Derm Venereol 2006;86:67-8. [PubMed]
- Shimauchi T, Kabashima K, Tokura Y. Solar urticaria as a manifestation of Churg-Strauss syndrome. Clin Exp Dermatol 2007;32:209-10. [Crossref] [PubMed]
- Skrapari I, Kagkelari E, Charitatos E, et al. Acute painless monocular visual loss due to central retinal artery occlusion in a patient with Churg-Strauss vasculitis. Clin Rheumatol 2008;27:125-7. [Crossref] [PubMed]
- Uehara M, Hashimoto T, Sasahara E, et al. Churg-Strauss syndrome presenting as myositis following unaccustomed exercise. J Clin Neurosci 2009;16:1232-3. [Crossref] [PubMed]
- Lestre S, Serrão V, João A, et al. Churg-Strauss syndrome presenting with cutaneous vasculitis. Acta Reumatol Port 2009;34:281-7. [PubMed]
- Vaglio A, Casazza I, Grasselli C, et al. Churg-Strauss syndrome. Kidney Int 2009;76:1006-11. [Crossref] [PubMed]
- Ishibashi M, Kudo S, Yamamoto K, et al. Churg-Strauss syndrome with coexistence of eosinophilic vasculitis, granulomatous phlebitis and granulomatous dermatitis in bullous pemphigoid-like blisters. J Cutan Pathol 2011;38:290-4. [Crossref] [PubMed]
- Gulati S, Patel NP, Swierczynski SL. Vasculitides associated with haematological malignancies: a case-based review. BMJ Case Rep 2012;2012:bcr2012007123. [Crossref] [PubMed]
- Kumano Y, Yoshida N, Fukuyama S, et al. Central retinal artery occlusion in a patient with ANCA-negative Churg-Strauss syndrome. Clin Ophthalmol 2012;6:1225-8. [Crossref] [PubMed]
- Tanaka K, Koga M, Ishibashi-Ueda H, et al. Churg-Strauss syndrome with concomitant occurrence of ischemic stroke and relapsing purpura. J Stroke Cerebrovasc Dis 2012;21:911.e9-10. [Crossref] [PubMed]
- Cheng MJ, Huang PH, Liao PW, et al. Multiple cerebral and cerebellar infarcts as the first clinical manifestation in a patient with Churg-Strauss syndrome: case report and literature review. Acta Neurol Taiwan 2012;21:169-75. [PubMed]
- Sulaiman W, Seung OP, Noor SM. Acute myocardial infarction as Eosinophilic granulomatosis with polyangiitis (formerly Churg Strauss syndrome) initial presentation. Rev Bras Reumatol 2014;54:393-6. [Crossref] [PubMed]
- Kawakami T, Shimosaka R, Takeuchi S, et al. Importance of appropriate location and frequency of biopsy for cutaneous manifestations in eosinophilic granulomatosis with polyangiitis. Int J Dermatol 2016;55:1388-90. [Crossref] [PubMed]
- Marques CC, Fernandes EL, Miquelin GM, et al. Cutaneous manifestations of Churg-Strauss syndrome: key to diagnosis. An Bras Dermatol 2017;92:56-8. [Crossref] [PubMed]
- Kuftinec G, Sami M, Aronowitz P. Asthma, Foot Drop, and Palpable Purpura in a Young Woman. J Gen Intern Med 2017;32:1405. [Crossref] [PubMed]
- Ferro JM. Vasculitis of the central nervous system. J Neurol 1998;245:766-76. [Crossref] [PubMed]
- Morgan JM, Raposo L, Gibson DG. Cardiac involvement in Churg-Strauss syndrome shown by echocardiography. Br Heart J 1989;62:462-6. [Crossref] [PubMed]
- Greco A, Rizzo MI, De Virgilio A, et al. Churg-Strauss syndrome. Autoimmun Rev 2015;14:341-8. [Crossref] [PubMed]
- Guillevin L, Pagnoux C, Seror R, et al. The Five-Factor Score revisited: assessment of prognoses of systemic necrotizing vasculitides based on the French Vasculitis Study Group (FVSG) cohort. Medicine (Baltimore) 2011;90:19-27. [Crossref] [PubMed]
- Moiseev S, Novikov P. Five Factor Score in patients with eosinophilic granulomatosis with polyangiitis (Churg-Strauss; EGPA): to use or not to use? Ann Rheum Dis 2014;73:e12. [Crossref] [PubMed]
- Mukhtyar C, Guillevin L, Cid MC, et al. EULAR recommendations for the management of primary small and medium vessel vasculitis. Ann Rheum Dis 2009;68:310-7. [Crossref] [PubMed]
- Comarmond C, Pagnoux C, Khellaf MFrench Vasculitis Study Group, et al. Eosinophilic granulomatosis with polyangiitis (Churg-Strauss): clinical characteristics and long-term follow-up of the 383 patients enrolled in the French Vasculitis Study Group cohort. Arthritis Rheum 2013;65:270-81. [Crossref] [PubMed]
Cite this article as: Msigwa SS, Li Y, Cheng X. An unusual concurrent dermatological and neurological manifestation in an elderly woman with eosinophilic granulomatosis with polyangiitis: a case report and literature review. J Xiangya Med 2020;5:41.


