
Metabolism and secretion mechanism of catecholamine syndrome and related treatment strategies
Introduction
Pheochromocytoma is a kind of tumor that originated in the adrenal medulla, the sympathetic ganglia and other parts of the chromaffin cells, secreting a large number of catecholamines (epinephrine, norepinephrine and dopamine) which act on the adrenergic receptors and cause high blood pressure (1). It is usually associated with paroxysmal or sustained hypertension, recurrent headaches, sweating, palpitations as well as weight loss (2,3). Severe cases can even be complicated by shock, heart failure, intracranial hemorrhage, ventricular fibrillation as well as myocardial infarction (4). If not diagnosed in time, the delaying treatment can cause serious damages in heart, blood vessel, brain and even death.
Pheochromocytoma is a kind of rare tumor. Its prevalence is not exactly known but has been estimated to be 1:6,500–1:2,500 in the United States (5). However, the autopsy results have even revealed that the prevalence was as high as 1:2,000, suggesting that many pheochromocytoma were not diagnosed before death (6). Besides, it is reported that the annual incidence is 2–10:100,000 individuals/year (7-9). Tumors can occur in all ages, but the highest incidence happens in 40 to 50 years old, with the basically same gender distribution (10-15).
The same as most tumors, the etiology of sporadic pheochromocytoma has not been fully explained, but the familial pheochromocytoma is related to heredity. The occurrence of pheochromocytoma is usually along with the mutations of PHD2, VHL, SDHx, IDH, HIF2A, MDH2 and FH which are involved in the hypoxic pathway as well as RET, NF1, KIF1Bβ, MAX and TMEM127 which are involved in the activating kinase signaling pathways. In addition, GDNF, GNAS, H-ras, K-ras, CDKN2A (p16), p53, BRCA1&2, BAP1, ATRX and KMT2D mutations also play roles in the development of pheochromocytoma (16-27).
The clinical manifestation of this disease is heterogeneous mainly related to predominate catecholamine secretions. Catecholamines can act on the heart, increasing heart rate, contractions, and blood pressure (28,29). Typical paroxysmal attack is often characterized by sudden high blood pressure even reaching 200–300/130–180 mmHg with severe headache, excess sweating and palpitations (30). Besides, prolonged, persistent hypertension can lead to left ventricular hypertrophy, cardiac enlargement and heart failure. As for metabolization, high concentration of epinephrine acts on central nervous system, especially sympathetic nervous system to make oxygen consumption increase and basal metabolization rate heighten, resulting in calorific and emaciation (31). Liver glycogen decomposition is accelerated and insulin secretion is inhibited to decrease glucose tolerance and increase liver glycogen dysplasia. For other performances, too many catecholamines reduce peristalsis and tension of the intestine, leading to constipation, intestinal dilatation, intestinal necrosis, bleeding and perforation. Under the action of large amounts of epinephrine, the blood cells are redistributed, making the white blood cell count in the peripheral blood increase, and sometimes the red blood cell may also increase (32).
Significant advances have been remarked in pheochromocytoma management since the tumor was first removed successfully by Roux and Mayo in 1926 (33). Before the use of α-adrenergic receptor blockers, the perioperative mortality was even as high as 50% in some researches (34,35) while after the introduction of α-blockers, the mortality range between 0–3% (33). Until now, surgical resection of tumors has been usually the first choice for the clinical use to control blood pressure and heart rate, treat arrhythmias, reduce circulating plasma volume, and prevent cardiovascular complications caused by excessive catecholamines in perioperative and intraoperative period (36,37). However, although treatments with α-blockers preoperatively seem to show some effect in many cases (38-40), limitations and side effects also appear along with them (41-47). Generally speaking, treatment therapies combining decreasing the producing of catecholamines upstream and the reception of catecholamines at the α-receptor level may show significant clinical effect in the treatment of pheochromocytoma.
Therefore, understanding the metabolism of catecholamines in pheochromocytoma and reducing its secretion by using drugs is a good way to guide the preoperative medication and treatment of pheochromocytoma. Our review summarized the literature to describe the synthesis and metabolic process of catecholamines in pheochromocytoma, the ion channels associated with catecholamine secretion and the influencing factors of the section of catecholamine. The related treatment strategies are then summarized based on the metabolism and secretion of catecholamine.
Pathways of catecholamines synthesis and metabolism in pheochromocytoma
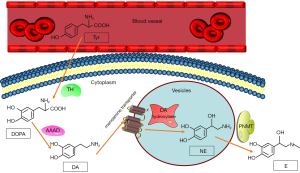
The first step in catecholamine synthesis is conversing tyrosine to 3,4-dihydroxyphenyl alanine (DOPA) by the rate-limiting enzyme, tyrosine hydroxylase (48). DOPA is then converted to dopamine by L-aromatic amino acid decarboxylase which is a kind of enzyme distributing widely in cells. After that, the vesicular monoamine transporter translocates dopamine into storage vesicles (49), in which dopamine β-hydroxylase converts dopamine to norepinephrine (50). Phenylethanolamine N-methyltransferase (PNMT) is an enzyme that primarily locate in the adrenal medullary chromaffin cells, and its action results in the conversion of norepinephrine to epinephrine (Figure 1). Since PNMT locates in cytoplasmic, the synthesis of epinephrine is dependent on the metabolism of norepinephrine, which leaks from the inner vesicles of the noradrenaline synthesis to the cytoplasm (51). Pheochromocytoma which produces catecholamines shows considerable differences in catecholamine levels based on biosynthetic enzyme expression (52-56). Most pheochromocytomas produce mainly norepinephrine, some produce norepinephrine and epinephrine, and few produce epinephrine. However, there are some extremely rare cases have been reported producing mainly dopamine (56,57).
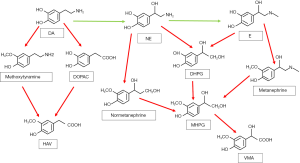
Catecholamines are metabolized by a variety of enzymes, such as monoamine oxidase (MAO), catechol-O methyltransferase (COMT), as well as sulfotransferase. There are also other enzymes participating glycol and acid deamination metabolites such as aldose or aldehyde reductase and aldehyde dehydrogenase. In addition, alcohol dehydrogenase has contribution to the formation of the final product of catecholamine metabolism. Thus, the multiple different metabolic pathways produce amounts of different metabolites (51).
MAO deamination stands for the more important one of the two major pathways on catecholamine metabolism. MAO has two isozymes, MAO-A and MAO-B, encoded by adjacent genes on the same chromosome (58). Both norepinephrine and epinephrine are metabolized by MAO in combination with aldose or aldehyde reductase into the deaminated glycol metabolite, 3,4 dihydroxyphenylglycol (DHPG). Besides, the aldehyde intermediate formed by the deamination of dopamine is preferentially metabolized by aldehyde dehydrogenase to 3,4 dihydroxyphenylacetic acid (DOPAC) (59-61).
The second major enzyme of catecholamine metabolism is COMT, which catalyze O-methylation of dopamine to methoxytyramine, epinephrine to metanephrine, and norepinephrine to normetanephrine. COMT also has two isozymes encoded by the same gene, which are membrane-bound and soluble COMT (62,63). Furthermore, the metabolites catalyzed by MAO can be metabolized by COMT sequentially. DHPG is metabolized to 3-methoxyl-4-hydroxyphenylglycol (MHPG) by COMT, while DOPAC can be metabolized to high homovanillic acid (HVA), which is the major end product of dopamine metabolism. Vanillic acid (VMA) is the major end product of human norepinephrine and epinephrine metabolism (64). VMA is mainly produced by MHPG oxidation, which is catalyzed by alcohol dehydrogenase (65-67). Except for the VMA, all catecholamines and their metabolites are metabolized into sulfate conjugates, representing other end products of catecholamine metabolism (51).
The activities of enzymes in catecholamine synthesis steps including tyrosine hydroxylase, L-aromatic amino acid decarboxylase and dopamine β-hydroxylase are found higher in pheochromocytoma than that in normal adrenal medulla, which may be the cause of excessive catecholamine production in pheochromocytoma (57,68,69). Another study found the similar results and came to the conclusion that catecholamines in the normal adrenal medulla may have a negative feedback mechanism through tyrosine hydroxylase, which not appears in pheochromocytoma. In addition, the increase in catecholamine degradation metabolism pathway of pheochromocytoma turns out to be unstable comparing with the normal adrenal medulla (70).
What’s more, in pheochromocytoma patients, more than 94% of elevated plasma metanephrines concentrations are caused by metabolism of catecholamines through COMT (71). The metabolism happens in pheochromocytoma tumor cells, rather than in the blood circulation by the extra-adrenal COMT (52). This suggests that in pheochromocytoma patients, most of the elevated levels of catecholamine metabolites are produced within the tumor rather than released outside the tumor (51) (See in Figure 2).
Ion channel and catecholamine secretion
Generally, the trigger for catecholamine secretion is the activity of the visceral nerve, which releases acetylcholine from nerve endings in the adrenal medulla, close enough to chromaffin cells that rapid synaptic potentials can be observed (72). The direct response to acetylcholine release is nicotinic receptors activation (73,74), which depolarizes chromaffin cells and allows Ca2+ to flow through the nicotinic receptors (75). This produces cellular depolarization, action potential discharges, as well as catecholamine secretion on the other hand (76).
Although neuro-induced catecholamine release is critical (77) the intrinsic electrical activity of catecholamines is also an important possibility that contributes to catecholamine secretion in some situations. Consistently, recent studies have revealed that chromaffin cells exhibit series of intrinsic excitatory patterns, including slow-wave burst which is potentially important for the secretion of catecholamines (78-83). Some ion channels are related to the secretion of catecholamine.
Na+ channels
Chromaffin cells in almost all mammalian species show obvious voltage-dependent Na+(Nav) current (83,84). It is reported that Na+ current in chromaffin cells is caused by Nav1.7 (85-87), while the evidence is still limited. The recent report shows that Nav1.3 and Nav1.7 both contribute to mouse chromaffin cells, with Nav1.3 subtype taking the predominance (83). However, the activation as well as the steady-state inactivation of Nav whole cell current seem to be consistent with only one type of channel (83). According to the research on the heterologous expression of Nav1.3 and Nav1.7, the two channels usually have similar functional characteristics, but compared with Nav1.7, the Nav1.3 semi-inactivated voltage usually shifts to the right (88,89). In mouse chromaffin cells, Nav current seems to be the most consistent with Nav1.3 channel (76).
Ca2+ channels
Chromaffin cells release catecholamines into the circulation via a calcium-dependent extracellular mechanism (90). Acetylcholine released by splanchnic-chromaffin cells causes cell depolarization and the opening of L- (91), N- (92), and P/Q-type (93) voltage-dependent calcium channels. Ca2+ enters the cell through these channels and triggers the fusion of vesicles with the plasma membrane, as well as releasing catecholamines (94). Therefore, the effectiveness of Ca2+ may be a possible control of the extent and rate of the secretion. ATP (95-97) and opioids (98) are co-released with catecholamines, inhibiting calcium channel currents through these three type channels (99-102) with a pathway delimited by G-protein-coupled membranes. This may form the basis of autoinhibitory mechanism for the entry of Ca2+, which has been shown to exist in different animal species (97,99-102), including human chromaffin cells (103). This Ca2+ entry control can therefore be used to modulate the release of catecholamines (Figure 3).
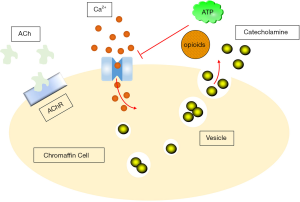
To illuminate the important Ca2+-dependent steps required for catecholamine secretion, chromaffin cells have been extensively studied to understand how cytosolic Ca2+ transients are coupled to exocytosis (104,105). Evidence differs from the different reports on whether a particular Ca2+ channel subtype plays a specific role in the coupling of catecholamine secretion, or exerts other subtype-specific effects (106-110).
In addition, the Ca2+ channel also takes a crucial part in regulating chromaffin cell excitability, which is triggered by the activation of inward and outward Ca2+ current, through the nearby BK channel or farther SK channel (111,112). It has been reported that there may exist a mechanism of tight coupling of Ca2+ and BK channels in some neuroendocrine cells. To support this suggestion, the P/Q-type channel and N-type channel have turned out to couple with BK channel activation (113,114). It seems that all Ca2+ channels can drive BK current in chromaffin cells, in which L-type may preferentially co-locate with it, especially when transient depolarization is in use (115,116). For longer depolarizing stimuli, secretion (117) and activation of the BK channels are both activated by a global elevation of Ca2+ from all Ca2+ channels (116,117).
The influencing factors of the section of catecholamine
The secretion of catecholamine and the accompanying hypertension are affected by many factors such as the triggers, tumor location, genetic background and so on. Catecholamines are stored in separate vesicles along with adenosine triphosphate, chromogranin and dopamine β-hydroxylase. Stress, pain, cold, heat, asphyxia, hypotension, hypoglycemia, and sympathetic excitation during hyponatremia increase the release of catecholamines (118). After preganglionic sympathetic excitation, the vesicle contents can be released by exocytosis (119). In addition, in some cases, catecholamines can be released not through sympathetic excitation and exocytosis.
Paraganglioma derived from extra-adrenal chromaffin tissues in sympathetic paravertebral ganglia of thoracic cavity, abdomen and pelvis. It also originates from parasympathetic ganglia located near the glossopharyngeal and vagus nerves in the neck and skull base (120) which is not able to produce catecholamines. About 80–85% of chromaffin-cell tumors are pheochromocytomas, while 15–20% are paragangliomas (121).
Pheochromocytoma has been proven to exhibit highly evident gene expression profiles in MEN2 and VHL syndrome which usually caused by the mutation of RET and VHL respectively (122,123). Although VHL tumors show activation of the hypoxia-angiogenic signaling pathway, the expression of many components associated with the catecholamine-related pathway is decreased compared to MEN2. For example, MEN2 tumors express phenylethanolamine N-methyltransferase, which converts norepinephrine to epinephrine and is not expressed in VHL tumors (56). The reason of the more symptomatic character of pheochromocytoma in MEN 2 than in VHL syndrome is considered to be the relative amount of norepinephrine and epinephrine produced by the two tumors and the different effects on α and β adrenergic receptors (56,124-126).
Furthermore, Tumors associated with SDHAF2, SDHC, and SDHD mutations are usually located in the head and neck, originating from the parasympathetic ganglia. As mentioned earlier, they often do not secrete catecholamines. As for NF1-related pheochromocytoma, it usually shows elevated norepinephrine and catecholamine metabolites (127). The biochemical features of SDHB mutant tumors are similar to those of norepinephrine predominance VHL patients, but they also show high methoxytyramine (a metabolite of dopamine) excretion as the increased biochemical marker (127). Last but not least, the biochemical features of tumors associated with SDHA, TMEM127, and MAX mutations have not been well determined (128).
What’s more, when cases like compression of the tumor during massage; direct trauma; eating foods rich in tyramine; and taking potentially stimulating drugs such as histamine, glucagon, tetraethylamine happen, high blood pressure may occur.
Treatment strategies for metabolism and secretion mechanisms
The treatment of pheochromocytoma with metyrosine (Table 1)
Table 1
| Author | Year | Study | Drugs | Efficacy |
|---|---|---|---|---|
| Renato Mariani-Costantini | 2019 | Paraganglioma: A Multidisciplinary Approach (129) | Metyrosine and α-blocker | Providing remarkable hemodynamic stability during operation |
| Mitsuhide Naruse | 2018 | Efficacy and Safety of Metyrosine in Pheochromocytoma/Paraganglioma: A Multi-center Trial in Japan (46) | Metyrosine and α-blocker | Improving symptoms of chronic excess catecholamine in metastatic and unresectable paraganglioma patients |
| Roger. R. Perry | 1990 | Surgical Management of Pheoclromocytoma with the Use of Metyrosine (33) | Metyrosine and phenoxybenzamine | Controlling blood pressure, reducing the blood loss and the need for intraoperative fluid replacement |
| Heather Wachtel | 2015 | Preoperative Metyrosine Improves Cardiovascular Outcomes for Patients Undergoing Surgery for Pheochromocytoma and Paraganglioma (130) | Metyrosine and phenoxybenzamine | Improving the hemodynamic stability during operation and decreasing the cardiovascular-specific complications rates |
| Jaime Steinsapir | 1997 | Metyrosine and Pheochromocytoma (131) | Metyrosine and phenoxybenzamine | Controlling blood pressure and reducing the need for antihypertensive drugs or pressor |
| Karl Engelman | 1968 | Biochemical and Pharmacologic Effects of a -Methyltyrosine in Man (132) | Metyrosine | A wide range of catecholamine synthesis reduction |
| Omar Serri | 1984 | Reduction in the Size of a Pheochromocytoma Pulmonary Metastasis (133) | Metyrosine | Shrinking the size of the functional metastasis in lung of a paraganglioma patients |
Metyrosine specifically inhibits tyrosine hydroxylase which catalyzes the conversion from tyrosine to DOPA, the first and rate-limiting step in the pathway of catecholamine synthesis (134). Clinical trials have proved that metyrosine can inhibit the synthesis of catecholamines thus improves the symptoms caused by catecholamine excess such as hypertension (132,135-138). In 1979, metyrosine was approved by the United States Food and Drug Administration for preoperative preparation of surgical patients, management of patients during surgical contraindications, and treatment of patients with metastatic pheochromocytoma (139). However, at that time, the clinical researches could neither meet the regulatory standards of evaluating the efficacy and safety, nor provide sufficient evidence for them (140). Metyrosine was recommended for long-term treatment for patients with metastatic pheochromocytoma in 1981 (141).
As described in the book Paraganglioma-A Multidisciplinary Approach, using metyrosine could provide remarkable hemodynamic stability during operation because of the inhibition of excessive catecholamine production, as a result, it can prevent potential fluctuations in blood pressure during tumor resection. Because the storage of catecholamines is usually exhausted within 3 days of surgery, for those with metastatic pheochromocytoma or high catecholamine levels, metyrosine is especially useful. Due to incomplete exhaustion of the catecholamines, no matter how much the dose is required, it is desirable to use metyrosine in combination with other α-blockers. This combination medication reduces the instability of blood pressure control, the blood loss and the need for volume replacement perioperatively, as compared to using α-blockers alone (129).
A Japanese study showed the efficacy and safety of metyrosine in patients with malignant and unresectable pheochromocytoma to improve symptoms related to catecholamine excess. It showed that the combination of metyrosine and α-blocker may be one of the optional treatments in pheochromocytoma patients (46).
Perry et al. reviewed 25 cases of consecutive patients undergoing pheochromocytoma surgery. Among them, 19 patients were prepared preoperatively with phenoxybenzamine and metyrosine while the other 6 patients were only given phenoxybenzamine. Although this study was a retrospective review rather than a prospective randomized trial, the results could explain that for surgical pheochromocytoma patients, management with both phenoxybenzamine and metyrosine showed a better performance than using phenoxybenzamine alone. The combined medication seemed to be able to better control blood pressure, reduce blood loss and reduce the need for intraoperative fluid replacement (33).
Another retrospective study investigated patients undergoing initial pheochromocytoma resection. One group was treated using metyrosine and phenoxybenzamine while the other using phenoxybenzamine only. It turned out that preoperative metyrosine improved the hemodynamic stability during operation and decreased the cardiovascular-specific complications rates in patients for pheochromocytoma resection. This report showed that the addition of metyrosine preoperatively may improve surgical outcomes (130).
In the study of Steinsapir et al., the combined use of a metyrosine and α-blocker showed a good result in better controlling blood pressure as well as reducing the need for antihypertensive drugs or pressor intraoperatively thus reducing the mortality of surgery. Using both medications would make patients with pheochromocytoma receive satisfactory hypertension treatment before operation (131).
From the data presented by Engelman et al., it was clear that metyrosine was useful in inhibiting catecholamine synthesis in human. What’s more, a wide range of catecholamine synthesis reduction lead to a remarkable improvement in pheochromocytoma patients’ clinical conditions. In some notable cases, metyrosine showed a better performance than other drugs, and seemed more desirable and simpler (132).
There was also a case report showing metyrosine effect on tumor progression. After long-term metyrosine treatment in a malignant pheochromocytoma patient, the size of the functional metastasis in lung shrunk (133). Because pheochromocytoma can also form spontaneous necrosis (142,143) metyrosine treatment may be accidental. However, because of no evidence to prove this phenomenon in pulmonary nodules, the conclusion was drawn that reducing the size of lung metastasis nodule may be due to the action of tyrosine (133).
Though with preferable usage, there are also some limitations reported in metyrosine. First of all, metyrosine is pretty expensive which limits its use in some countries. The cost has increased dramatically which makes the access and availability to this medication limitary. Secondly, the main side effects usually include symptoms in the central or peripheral nervous systems, because metyrosine can cross the blood-brain barrier and inhibit catecholamine synthesis. Other side effects usually include anxiety, sedation, fatigue, depression, lethargy, crystalluria and gastrointestinal manifestations like diarrhea (144-146).
The treatment of pheochromocytoma with calcium channel blockers
Catecholamine secretion is caused by an increase in intracellular Ca2+ concentration, which is a result of increased cell membrane permeability to extracellular Ca2+. A variety of factors influence the Ca2+-mediated catecholamine release process (147-150).
In chromaffin tissues, chromaffin cells release catecholamines through a Ca2+-dependent extracellular mechanism (90). Acetylcholine releases in synapse of visceral chromaffin cells, causing cell depolarization and opening voltage-dependent Ca2+ channels (91-93). After that, extracellular Ca2+ enters through these channels, triggers the fusion of secretory vesicles with the plasma membrane and releases catecholamines (94).
Under pressure conflict, normal cells show a highly controlled secretory response, while tumor cells begin to secrete in an unsynchronized and uncontrolled manner, producing a large amount of catecholamines into the circulation, resulting in the typical symptoms suffered by pheochromocytoma patients. In these cases, using calcium channel blockers, which include amlodipine, nicardipine, verapamil and nifedipine is an effective method (147).
Calcium channel blockers is commonly used for patents with hypertension and maintain the blood pressure by decreasing the pressure of peripheral vessels. While in some studies, calcium channel blockers may inhibit norepinephrine-mediated calcium fluxes into vascular smooth muscle cells for the purpose of controlling blood pressure and arrhythmia. They also prevent catecholamine-related coronary artery spasm and help improve cardiac function (151) without causing orthostatic hypotension (152-154). Due to the pharmacological action of the calcium antagonist, its use alone does not improve all the hemodynamic changes brought by pheochromocytoma, and only in the following three conditions, calcium channel blockers can be used combining with or replacing the α-blocker (155,156), (I) when single use of α-blocker, blood pressure control is not satisfactory, calcium channel blockers can be used in combination to improve efficacy. Besides, the dose of α-blocker can be reduced; (II) when patients cannot tolerate α-blockers having serious side effects, it can be replaced by calcium channel blockers; (III) when blood pressure is normal or only intermittently elevated, calcium channel blockers can replace α-blocker to prevent hypotension or orthostatic hypotension.
Use of magnesium sulfate during the perioperative period of pheochromocytoma
Magnesium sulfate is mainly used for perioperative hypertension or anesthesia. The mechanism of magnesium sulfate to lower blood pressure is mainly: (I) relaxation of vascular smooth muscle and expansion of vascular wall, (II) inhibition of adrenal medulla and adrenergic nerve endings to secrete catecholamine, and (III) direct inhibition of catecholamine receptors (157).
Reducing tyrosine-rich food intake
Tyrosine is one of the raw materials for the synthesis of catecholamines. Preoperative reduction of tyrosine-rich food intake may be important in reducing the increase of blood pressure caused by intraoperative catecholamine secretion. Tyrosine-rich foods include pickled fish, milk, lactic acid drinks, cheese, animal liver, beef, fermented food, broad beans, as well as beer.
Conclusion and expectations
Synthesis, conversion, release, as well as type of catecholamines produced are heterogeneous among patients with pheochromocytoma. These differences in catecholamine precursors, metabolites and their accompanying variations can offer useful information about pheochromocytoma, which includes potential mutations, locations either inside or outside the adrenal gland, tumor size and the degree of metastasis (51).
Catecholamines are catalyzed by tyrosine via tyrosine hydroxylase to produce DOPA, and then gradually reacts to produce dopamine, norepinephrine and epinephrine. Catecholamines are metabolized mainly through MAO and COMT pathways. Understanding its related characteristics and pay attention to the protection show good effect on the nursing and treatment of pheochromocytoma.
Preoperative pretreatment with α-blockers was routine before pheochromocytoma surgery, which showed a good result in improving the perioperative progression and reducing arrhythmias (38-40). However, there are a lot of limitations in the use of α-blockers. In our review, we summarized the articles that tried the therapies using metyrosine or combining α-blocker and metyrosine, the results showed remarkable clinical effect in the treatment of pheochromocytoma. However, there are still some limitations in the use of metyrosine and the clinical trials are insufficient. More prospective randomized trials need to be done to provide more evidence proving the effect of metyrosine.
When the cell membrane increases the permeability of extracellular Ca2+ by different causes, the intracellular Ca2+ concentration increases and the catecholamine is secreted afterward. Calcium channel blockers show good effect in these cases and can use in company with or replace the α-blocker. Magnesium sulfate can also be used for perioperative hypotension or anesthesia with the mechanism of relaxing vascular smooth muscle, inhibiting catecholamine secretion, and inhibiting catecholamine receptors.
As for catecholamine metabolism, there are still many unexplained mechanisms and many potential metabolic targets. For example, do different gene mutations cause different levels of catecholamine secretion, and what are the mechanisms involved? There are many important enzymes in catecholamine metabolism pathway. Can useful drugs be developed to inhibit the production of catecholamines or accelerate their metabolism so as to reduce the incidence of hypertension in patients with pheochromocytoma before operation? These are expected to be addressed in future studies.
Acknowledgments
Funding: None.
Footnote
Peer Review File: Available at http://dx.doi.org/10.21037/jxym-20-88
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jxym-20-88). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Fonseca V, Bouloux PM. Phaeochromocytoma and paraganglioma. Baillieres Clin Endocrinol Metab 1993;7:509-44. [Crossref] [PubMed]
- Cotesta D, Petramala L, Serra V, et al. Clinical Experience with Pheochromocytoma in a Single Centre Over 16 Years. High Blood Press Cardiovasc Prev 2009;16:183-93. [Crossref] [PubMed]
- Kopetschke R, Slisko M, Kilisli A, et al. Frequent incidental discovery of phaeochromocytoma: data from a German cohort of 201 phaeochromocytoma. Eur J Endocrinol 2009;161:355-61. [Crossref] [PubMed]
- James MF, Larissa C. Pheochromocytoma crisis: the use of magnesium sulfate. Anesth Analg 2004;99:680-6. table of contents. [Crossref] [PubMed]
- Chen H, Sippel RS, O'Dorisio MS, et al. The North American Neuroendocrine Tumor Society consensus guideline for the diagnosis and management of neuroendocrine tumors: pheochromocytoma, paraganglioma, and medullary thyroid cancer. Pancreas 2010;39:775-83. [Crossref] [PubMed]
- McNeil AR, Blok BH, Koelmeyer TD, et al. Phaeochromocytomas discovered during coronial autopsies in Sydney, Melbourne and Auckland. Aust N Z J Med 2000;30:648-52. [Crossref] [PubMed]
- Beard CM, Sheps SG, Kurland LT, et al. Occurrence of pheochromocytoma in Rochester, Minnesota, 1950 through 1979. Mayo Clin Proc 1983;58:802-4. [PubMed]
- Stenstr MG, Sv Rdsudd K. Pheochromocytoma in Sweden 1958-1981. An analysis of the National Cancer Registry Data. J Intern Med 2010;220:225-32.
- Ariton M, Juan CS. AvRuskin TW. Pheochromocytoma: clinical observations from a Brooklyn tertiary hospital. Endocr Pract 2000;6:249-52. [Crossref] [PubMed]
- O'Riordain DS, Young WF Jr, et al. Clinical spectrum and outcome of functional extraadrenal paraganglioma. World J Surg 1996;20:916-21; discussion 922. [Crossref] [PubMed]
- Goldstein RE, O'Neill JA Jr, Holcomb GW 3rd, et al. Clinical experience over 48 years with pheochromocytoma. Ann Surg 1999;229:755-64; discussion 764-6. [Crossref] [PubMed]
- Erickson D, Kudva YC, Ebersold MJ, et al. Benign Paragangliomas: Clinical Presentation and Treatment Outcomes in 236 Patients. J Clin Endocrinol Metab 2001;86:5210. [Crossref] [PubMed]
- Favia G, Lumachi F, Polistina F, et al. Pheochromocytoma, a rare cause of hypertension: long-term follow-up of 55 surgically treated patients. World J Surg 1998;22:689-93; discussion 694. [Crossref] [PubMed]
- Cascón A, Pita G, Burnichon N, et al. Genetics of pheochromocytoma and paraganglioma in Spanish patients. J Clin Endocrinol Metab 2009;94:1701-5. [Crossref] [PubMed]
- Mannelli M, Castellano M, Schiavi F, et al. Clinically guided genetic screening in a large cohort of italian patients with pheochromocytomas and/or functional or nonfunctional paragangliomas. J Clin Endocrinol Metab 2009;94:1541. [Crossref] [PubMed]
- Pillai S, Gopalan V, Smith RA, et al. Updates on the genetics and the clinical impacts on phaeochromocytoma and paraganglioma in the new era. Crit Rev Oncol Hematol 2016;100:190-208. [Crossref] [PubMed]
- Comino-Méndez I, Gracia-Aznárez FJ, Schiavi F, et al. Exome sequencing identifies MAX mutations as a cause of hereditary pheochromocytoma. Nat Genet 2011;43:663-7. [Crossref] [PubMed]
- Castro-Vega LJ, Buffet A, De Cubas AA, et al. Germline mutations in FH confer predisposition to malignant pheochromocytomas and paragangliomas. Hum Mol Genet 2014;23:2440-6. [Crossref] [PubMed]
- Qin Y, Yao L, King EE, et al. Germline mutations in TMEM127 confer susceptibility to pheochromocytoma. Nat Genet 2010;42:229-33. [Crossref] [PubMed]
- Mulligan LM, Kwok JBJ, Healey CS, et al. Germ-line mutations of the RET proto-oncogene in multiple endocrine neoplasia type 2A. Nature 1993;363:458-60. [Crossref] [PubMed]
- Niemann S, Müller U. Mutations in SDHC cause autosomal dominant paraganglioma, type 3. Nat Genet 2000;26:268-70. [Crossref] [PubMed]
- Baysal BE, Ferrell RE, Willett-Brozick JE, et al. Mutations in SDHD, a mitochondrial complex II gene, in hereditary paraganglioma. Science 2000;287:848-51. [Crossref] [PubMed]
- Hao HX, Khalimonchuk O, Schraders M, et al. SDH5, a gene required for flavination of succinate dehydrogenase, is mutated in paraganglioma. Science 2009;325:1139-42. [Crossref] [PubMed]
- Burnichon N, Brière JJ, Libé R, et al. SDHA is a tumor suppressor gene causing paraganglioma. Hum Mol Genet 2010;19:3011-20. [Crossref] [PubMed]
- Astuti D, Latif F, Dallol A, et al. Gene mutations in the succinate dehydrogenase subunit SDHB cause susceptibility to familial pheochromocytoma and to familial paraganglioma. Am J Hum Genet 2001;69:49-54. [Crossref] [PubMed]
- Crona J, Delgado Verdugo A, Maharjan R, et al. Somatic mutations in H-RAS in sporadic pheochromocytoma and paraganglioma identified by exome sequencing. J Clin Endocrinol Metab 2013;98:E1266-71. [Crossref] [PubMed]
- Cascón A, Comino-Méndez I, Currás-Freixes M, et al. Whole-exome sequencing identifies MDH2 as a new familial paraganglioma gene. J Natl Cancer Inst 2015;107:djv053. [Crossref] [PubMed]
- Wang X, Pei J, Hu X. The Brain-Heart Connection in Takotsubo Syndrome: The Central Nervous System, Sympathetic Nervous System, and Catecholamine Overload. Cardiol Res Pract 2020;2020:4150291. [Crossref] [PubMed]
- Byrne CJ, Khurana S, Kumar A, et al. Inflammatory Signaling in Hypertension: Regulation of Adrenal Catecholamine Biosynthesis. Front Endocrinol (Lausanne) 2018;9:343. [Crossref] [PubMed]
- Reisch N, Peczkowska M, Januszewicz A, et al. Pheochromocytoma: presentation, diagnosis and treatment. J Hypertens 2006;24:2331-9. [Crossref] [PubMed]
- Tank AW, Lee Wong D. Peripheral and central effects of circulating catecholamines. Compr Physiol 2015;5:1-15. [PubMed]
- Aygun N, Uludag M. Pheochromocytoma and Paraganglioma: From Epidemiology to Clinical Findings. Sisli Etfal Hastan Tip Bul 2020;54:159-68. [Crossref] [PubMed]
- Perry RR, Keiser HR, Norton JA, et al. Surgical management of pheochromocytoma with the use of metyrosine. Ann Surg 1990;212:621-8. [Crossref] [PubMed]
- Levine SN, Mcdonald JC. The evaluation and management of pheochromocytomas. Adv Surg 1984;17:281-313. [PubMed]
- Pullerits J, Ein S, Balfe JW. Anaesthesia for phaeochromocytoma. Can J Anaesth 1988;35:526-34. [Crossref] [PubMed]
- Clark OH, Benson AB, Berlin JD, et al. NCCN Clinical Practice Guidelines in Oncology: neuroendocrine tumors. J Natl Compr Canc Netw 2009;7:712. [Crossref] [PubMed]
- Lenders JWM, Quan-Yang D, Graeme E, et al. Pheochromocytoma and paraganglioma: an endocrine society clinical practice guideline. J Clin Endocrinol Metab 2014;99:1915. [Crossref] [PubMed]
- Walther MM, Keiser HR, Linehan WM. Pheochromocytoma: evaluation, diagnosis, and treatment. World J Urol 1999;17:35. [Crossref] [PubMed]
- Dabrowska B, Pruszczyk P, Dabrowski A, et al. Influence of alpha-adrenergic blockade on ventricular arrhythmias, QTc interval and heart rate variability in phaeochromocytoma. J Hum Hypertens 1995;9:925-9. [PubMed]
- Russell WJ, Metcalfe IR, Tonkin AL, et al. The preoperative management of phaeochromocytoma. Anaesth Intensive Care 1998;26:196-200. [Crossref] [PubMed]
- Nickerson M. The pharmacology of adrenergic blockade. J Pharmacol Exp Ther 1949;95:27. [PubMed]
- Salem MR, Ivankovic AD. Management of phentolamine-resistant phaeochromocytoma with beta-adrenergic blockade. A case report. Br J Anaesth 1969;41:1087-90. [Crossref] [PubMed]
- Berthelsen S, Pettinger WA. A functional basis for classification of alpha-adrenergic receptors. Life Sci 1977;21:595-606. [Crossref] [PubMed]
- Modlinger RS, Ertel NH, Hauptman JB. Adrenergic blockade in pheochromocytoma. Arch Intern Med 1983;143:2245-6. [Crossref] [PubMed]
- Hauptman JB, Modlinger RS, Ertel NH. Pheochromocytoma resistant to alpha-adrenergic blockade. Arch Intern Med 1983;143:2321-3. [Crossref] [PubMed]
- Naruse M, Satoh F, Tanabe A, et al. Efficacy and safety of metyrosine in pheochromocytoma/paraganglioma: a multi-center trial in Japan. Endocr J 2018;65:359-71. [Crossref] [PubMed]
- Mullen JP, Cartwright RC, Tisherman SE, et al. Pathogenesis and pharmacologic management of pseudo-obstruction of the bowel in pheochromocytoma. Am J Med Sci 1985;290:155-8. [Crossref] [PubMed]
- Nagatsu T, Levitt M, Udenfriend S. Tyrosine hydroxylase. the initial step in norepinephrine biosynthesis. J Biol Chem 1964;239:2910-7. [PubMed]
- Henry JP, Sagné C, Bedet C, et al. The vesicular monoamine transporter: from chromaffin granule to brain. Neurochem Int 1998;32:227-46. [Crossref] [PubMed]
- Ahn NG, Klinman JP. Nature of rate-limiting steps in a compartmentalized enzyme system. Quantitation of dopamine transport and hydroxylation rates in resealed chromaffin granule ghosts. J Biol Chem 1989;264:12259. [PubMed]
- Eisenhofer G, Huynh TT, Hiroi M, et al. Understanding catecholamine metabolism as a guide to the biochemical diagnosis of pheochromocytoma. Rev Endocr Metab Disord 2001;2:297-311. [Crossref] [PubMed]
- Crout JR, Sjoerdsma A. Turnover and metabolism of catecholamines in patients with pheochromocytoma. J Clin Investig 1964;43:94-102. [Crossref] [PubMed]
- Feldman JM. Phenylethanolamine-N-methyltransferase activity determines the epinephrine concentration of pheochromocytomas. Res Commun Chem Pathol Pharmacol 1981;34:389-98. [PubMed]
- Osamura RY, Yasuda O, Kawai K, et al. Immunohistochemical localization of catecholamine-synthesizing enzymes in human pheochromocytomas. Endocr Pathol 1990;1:102-8. [Crossref] [PubMed]
- Kimura N, Miura Y, Nagatsu I, et al. Catecholamine synthesizing enzymes in 70 cases of functioning and non-functioning phaeochromocytoma and extra-adrenal paraganglioma. Virchows Arch A Pathol Anat Histopathol 1992;421:25-32. [Crossref] [PubMed]
- Eisenhofer G, Walther MM, Huynh TT, et al. Pheochromocytomas in von Hippel-Lindau syndrome and multiple endocrine neoplasia type 2 display distinct biochemical and clinical phenotypes. J Clin Endocrinol Metab 2001;86:1999-2008. [Crossref] [PubMed]
- Yasunari K, Kohno M, Minami M, et al. A dopamine-secreting pheochromocytoma. J Cardiovasc Pharmacol 2000;36:S75. [Crossref] [PubMed]
- Weyler W, Hsu YPP, Breakafield XO. Biochemistry and genetics of monoamine oxidase. Pharmacol Ther 1990;47:391-417. [Crossref] [PubMed]
- Kawamura M, Kopin IJ, Kador PF, et al. Effects of aldehyde/aldose reductase inhibition on neuronal metabolism of norepinephrine. J Auton Nerv Syst 1997;66:145. [Crossref] [PubMed]
- Kawamura M, Eisenhofer G, Kopin IJ, et al. Aldose reductase, a key enzyme in the oxidative deamination of norepinephrine in rats. Biochem Pharmacol 1999;58:517. [Crossref] [PubMed]
- Duncan RJ, Sourkes TL. Some enzymic aspects of the production of oxidized or reduced metabolites of catecholamines and 5-hydroxytryptamine by brain tissues. J Neurochem 1974;22:663-9. [Crossref] [PubMed]
- Lotta T, Vidgren JC, Ulmanen I, et al. Kinetics of human soluble and membrane-bound catechol O-methyltransferase: a revised mechanism and description of the thermolabile variant of the enzyme. Biochemistry 1995;34:4202-10. [Crossref] [PubMed]
- Roth JA. Membrane-bound catechol-O-methyltransferase: A reevaluation of its role in the O-methylation of the catecholamine neurotransmitters. Rev Physiol Biochem Pharmacol 1992;120:1-29. [Crossref] [PubMed]
- Eisenhofer G, Pecorella W, Pacak K, et al. The neuronal and extraneuronal origins of plasma 3-methoxy-4-hydroxyphenylglycol in rats. J Auton Nerv Syst 1994;50:93-107. [Crossref] [PubMed]
- Blombery PA, Kopin IJ, Gordon EK, et al. Conversion of MHPG to vanillylmandelic acid. Implications for the importance of urinary MHPG. Arch Gen Psychiatr 1980;37:1095-8. [Crossref] [PubMed]
- Mårdh G, Sjöquist B, Anggård E. Norepinephrine metabolism in man using deuterium labeling: turnover 4-hydroxy-3-methoxymandelic acid. J Neurochem 1982;38:1582-7. [Crossref] [PubMed]
- Eisenhofer G, Aneman A, Hooper D, et al. Mesenteric organ production, hepatic metabolism, and renal elimination of norepinephrine and its metabolites in humans. J Neurochem 1996;66:1565-73. [Crossref] [PubMed]
- Nagatsu T, Mizutani K, Sudo Y, et al. Tyrosine hydroxylase in human adrenal glands and human pheochromocytoma. Clin Chim Acta 1972;39:417-24. [Crossref] [PubMed]
- Jarrott B, Louis WJ. Abnormalities in enzymes involved in catecholamine synthesis and catabolism in phaeochromocytoma. Clin Sci Mol Med 1977;53:529-35. [Crossref] [PubMed]
- Nakada T, Furuta H, Katayama T. Catecholamine metabolism in pheochromocytoma and normal adrenal medullae. J Urol 1988;140:1348-51. [Crossref] [PubMed]
- Eisenhofer G, Keiser H, Friberg P, et al. Plasma metanephrines are markers of pheochromocytoma produced by catechol-O-methyltransferase within tumors. J Clin Endocrinol Metab 1998;83:2175-85. [Crossref] [PubMed]
- Kajiwara R, Sand O, Kidokoro Y, et al. Functional organization of chromaffin cells and cholinergic synaptic transmission in rat adrenal medulla. Jpn J Physiol 1997;47:449. [Crossref] [PubMed]
- Fenwick EM, Marty A, Neher E. A patch-clamp study of bovine chromaffin cells and of their sensitivity to acetylcholine. J Physiol 1982;331:577-97. [Crossref] [PubMed]
- Role LW, Perlman RL. Both nicotinic and muscarinic receptors mediate catecholamine secretion by isolated guinea-pig chromaffin cells. Neuroscience 1983;10:979-85. [Crossref] [PubMed]
- Zhou Z, Neher E. Calcium permeability of nicotinic acetylcholine receptor channels in bovine adrenal chromaffin cells. Pflugers Arch 1993;425:511. [Crossref] [PubMed]
- Lingle CJ, Martinez-Espinosa PL, Guarina L, et al. Roles of Na+, Ca2+, and K+ channels in the generation of repetitive firing and rhythmic bursting in adrenal chromaffin cells. Pflugers Arch 2018;470:39-52. [Crossref] [PubMed]
- de Diego AM, Gandía L, García AG. A physiological view of the central and peripheral mechanisms that regulate the release of catecholamines at the adrenal medulla. Acta Physiol (Oxf) 2008;192:287-301. [Crossref] [PubMed]
- Albiñana E, Segura-Chama P, Baraibar AM, et al. Different contributions of calcium channel subtypes to electrical excitability of chromaffin cells in rat adrenal slices. J Neurochem 2015;133:511-21. [Crossref] [PubMed]
- Guarina L, Vandael DH, Carabelli V, et al. Low pHo boosts burst firing and catecholamine release by blocking TASK-1 and BK channels while preserving Cav1 channels in mouse chromaffin cells. J Physiol 2017;595:2587. [Crossref] [PubMed]
- Gullo F, Ales E, Rosati B, et al. ERG K+ channel blockade enhances firing and epinephrine secretion in rat chromaffin cells: the missing link to LQT2-related sudden death? FASEB J 2003;17:330-2. [Crossref] [PubMed]
- Marcantoni A, Baldelli P, Hernandez-Guijo JM, et al. L-type calcium channels in adrenal chromaffin cells: role in pace-making and secretion. Cell Calcium 2007;42:397-408. [Crossref] [PubMed]
- Martinez-Espinosa PL, Yang C, Gonzalez-Perez V, et al. Knockout of the BK β2 subunit abolishes inactivation of BK currents in mouse adrenal chromaffin cells and results in slow-wave burst activity. J Gen Physiol 2014;144:275. [Crossref] [PubMed]
- Vandael DHF, Ottaviani MM, Christian L, et al. Reduced availability of voltage-gated sodium channels by depolarization or blockade by tetrodotoxin boosts burst firing and catecholamine release in mouse chromaffin cells. J Physiol 2015;593:905-27. [Crossref] [PubMed]
- Lou XL, Yu X, Chen XK, et al. Na+ channel inactivation: a comparative study between pancreatic islet beta-cells and adrenal chromaffin cells in rat. J Physiol 2003;548:191-202. [Crossref] [PubMed]
- Klugbauer N, Lacinova L, Flockerzi V, et al. Structure and functional expression of a new member of the tetrodotoxin-sensitive voltage-activated sodium channel family from human neuroendocrine cells. EMBO J 1995;14:1084-90. [Crossref] [PubMed]
- Wada A, Wanke EF, Schiavon E. Voltage-dependent Na(v)1.7 sodium channels: multiple roles in adrenal chromaffin cells and peripheral nervous system. Acta Physiol (Oxf) 2008;192:221-31. [Crossref] [PubMed]
- Wada A, Yanagita T, Yokoo H, et al. Regulation of cell surface expression of voltage-dependent Nav1.7 sodium channels: mRNA stability and posttranscriptional control in adrenal chromaffin cells. Front Biosci 2004;9:1954. [Crossref] [PubMed]
- Cummins TR, Aglieco F, Renganathan M, et al. Nav1.3 sodium channels: rapid repriming and slow closed-state inactivation display quantitative differences after expression in a mammalian cell line and in spinal sensory neurons. J Neurosci 2001;21:5952-61. [Crossref] [PubMed]
- Herzog RI, Cummins TR, Farshid G, et al. Distinct repriming and closed-state inactivation kinetics of Nav1.6 and Nav1.7 sodium channels in mouse spinal sensory neurons. J Physiol 2003;551:741-50. [Crossref] [PubMed]
- Douglas WW, Rubin RP. The role of calcium in the secretory response of the adrenal medulla to acetylcholine. J Physiol 1961;159:40-57. [Crossref] [PubMed]
- García AG, Sala F, Reig JA, et al. Dihydropyridine BAY-K-8644 activates chromaffin cell calcium channels. Nature 1984;309:69-71. [Crossref] [PubMed]
- Ballesta JJ, Palmero M, Hidalgo MJ, et al. Separate binding and functional sites for omega-conotoxin and nitrendipine suggest two types of calcium channels in bovine chromaffin cells. J Neurochem 1989;53:1050-6. [Crossref] [PubMed]
- López MG, Villarroya M, Lara B, et al. Q- and L-type Ca2+ channels dominate the control of secretion in bovine chromaffin cells. FEBS Lett 1994;349:331-7. [Crossref] [PubMed]
- Neher E. Vesicle pools and Ca2+ microdomains: new tools for understanding their roles in neurotransmitter release. Neuron 1998;20:389-99. [Crossref] [PubMed]
- Diverse-Pierluissi M, Dunlap K, Westhead EW. Multiple actions of extracellular ATP on calcium currents in cultured bovine chromaffin cells. Proc Natl Acad Sci U S A 1991;88:1261-5. [Crossref] [PubMed]
- Lim W, Kim SJ, Yan HD, et al. Ca2+-channel-dependent and -independent inhibition of exocytosis by extracellular ATP in voltage-clamped rat adrenal chromaffin cells. Pflugers Arch 1997;435:34-42. [Crossref] [PubMed]
- Otstuguro K, Ohta T, Ito S, et al. Modulation of calcium current by ATP in guinea-pig adrenal chromaffin cells. Pflugers Arch 1996;431:402. [Crossref] [PubMed]
- Twitchell WA, Rane SG. Opioid peptide modulation of Ca(2+)-dependent K+ and voltage-activated Ca2+ currents in bovine adrenal chromaffin cells. Neuron 1993;10:701-9. [Crossref] [PubMed]
- Albillos A, Gandía L, Michelena P, et al. The mechanism of calcium channel facilitation in bovine chromaffin cells. J Physiol 1996;494:687-95. [Crossref] [PubMed]
- Carabelli V, Carra I, Carbone E. Localized Secretion of ATP and Opioids Revealed through Single Ca 2+ Channel Modulation in Bovine Chromaffin Cells. Neuron 1998;20:1255-68. [Crossref] [PubMed]
- Currie KP, Fox AP. ATP serves as a negative feedback inhibitor of voltage-gated Ca2+ channel currents in cultured bovine adrenal chromaffin cells. Neuron 1996;16:1027. [Crossref] [PubMed]
- Hernández-Guijo JM, Carabelli V, Gandía L, et al. Voltage-independent autocrine modulation of L-type channels mediated by ATP, opioids and catecholamines in rat chromaffin cells. Eur J Neurosci 1999;11:3574-84. [Crossref] [PubMed]
- Gandía L, Mayorgas I, Michelena P, et al. Human adrenal chromaffin cell calcium channels: drastic current facilitation in cell clusters, but not in isolated cells. Pflugers Arch 1998;436:696-704. [Crossref] [PubMed]
- Duan K, Yu X, Zhang C, et al. Control of secretion by temporal patterns of action potentials in adrenal chromaffin cells. J Neurosci 2003;23:11235-43. [Crossref] [PubMed]
- García AG, García-De-Diego AM, Luis G, et al. Calcium signaling and exocytosis in adrenal chromaffin cells. Physiol Rev 2006;86:1093-131. [Crossref] [PubMed]
- Albillos A, Neher E, Moser T. R-Type Ca2+ channels are coupled to the rapid component of secretion in mouse adrenal slice chromaffin cells. J Neurosci 2000;20:8323. [Crossref] [PubMed]
- Álvarez YD, Belingheri AV, Perez Bay AE, et al. The immediately releasable pool of mouse chromaffin cell vesicles is coupled to P/Q-type calcium channels via the synaptic protein interaction site. PLoS One 2013;8:e54846. [Crossref] [PubMed]
- Álvarez YD, Ibañez LI, Uchitel OD, et al. P/Q Ca channels are functionally coupled to exocytosis of the immediately releasable pool in mouse chromaffin cells. Cell Calcium 2008;43:155-64. [Crossref] [PubMed]
- Carabelli V, Marcantoni A, Comunanza V, et al. Fast exocytosis mediated by T- and L-type channels in chromaffin cells: distinct voltage-dependence but similar Ca2+-dependence. Eur Biophys J 2007;36:753. [Crossref] [PubMed]
- Giancippoli A, Novara M, de Luca A, et al. Low-threshold exocytosis induced by cAMP-recruited CaV3.2 (alpha1H) channels in rat chromaffin cells. Biophys J 2006;90:1830-41. [Crossref] [PubMed]
- Neely A, Lingle CJ. Two components of calcium-activated potassium current in rat adrenal chromaffin cells. J Physiol 1992;453:97-131. [Crossref] [PubMed]
- Vandael DH, Marcantoni A, Carbone E. Cav1.3 Channels as Key Regulators of Neuron-Like Firings and Catecholamine Release in Chromaffin Cells. Curr Mol Pharmacol 2015;8:149-61. [Crossref] [PubMed]
- Marrion NV, Tavalin SJ. Selective activation of Ca2+-activated K+ channels by co-localized Ca2+ channels in hippocampal neurons. Nature 1998;395:900-5. [Crossref] [PubMed]
- Womack MD, Carolyn C, Kamran K. Calcium-activated potassium channels are selectively coupled to P/Q-type calcium channels in cerebellar Purkinje neurons. J Neurosci 2004;24:8818. [Crossref] [PubMed]
- Marcantoni A, Dhmahapatra V. Loss of Cav1.3 channels reveals the critical role of L-type and BK channel coupling in pacemaking mouse adrenal chromaffin cells. J Neurosci 2010;30:491-504. [Crossref] [PubMed]
- Prakriya M, Lingle CJ. BK channel activation by brief depolarizations requires Ca2+ influx through L- and Q-type Ca2+ channels in rat chromaffin cells. J Neurophysiol 1999;81:2267-78. [Crossref] [PubMed]
- Lukyanetz EA, Neher E. Different types of calcium channels and secretion from bovine chromaffin cells. Eur J Neurosci 1999;11:2865-73. [Crossref] [PubMed]
- Lewis MJ. Proceedings: Delta-tetrahydrocannabinol and adrenergic mechanisms. Br J Pharmacol 1975;54:277P. [PubMed]
- Ryan US, Ryan JW, Smith DS, et al. Fenestrated endothelium of the adrenal gland: freeze-fracture studies. Tissue Cell 1975;7:181-90. [Crossref] [PubMed]
- Delellis RA. Pathology and Genetics of Tumours of Endocrine Organs. World Health Organization 2004.
- Lenders JW, Eisenhofer G, Mannelli M, et al. Phaeochromocytoma. Lancet 2005;366:665-75. [Crossref] [PubMed]
- Eisenhofer G, Huynh TT, Pacak K, et al. Distinct gene expression profiles in norepinephrine- and epinephrine-producing hereditary and sporadic pheochromocytomas: activation of hypoxia-driven angiogenic pathways in von Hippel-Lindau syndrome. Endocr Relat Cancer 2004;11:897-911. [Crossref] [PubMed]
- Neumann HPH, Young WF Jr, Eng C. Pheochromocytoma and Paraganglioma. N Engl J Med 2019;381:552-65. [Crossref] [PubMed]
- Ito Y, Fuimoto Y, Obara T. The role of epinephrine, norepinephrine, and dopamine in blood pressure disturbances in patients with pheochromocytoma. World J Surg 1992;16:759. [Crossref] [PubMed]
- Lance JW, Hinterberger H. Symptoms of pheochromocytoma, with particular reference to headache, correlated with catecholamine production. Arch Neurol 1976;33:281-8. [Crossref] [PubMed]
- Aronoff SL, Passamani E, Borowsky BA, et al. Norepinephrine and epinephrine secretion from a clinically epinephrine-secreting pheochromocytoma. Am J Med 1980;69:321-4. [Crossref] [PubMed]
- Eisenhofer G, Lenders JW, Timmers H, et al. Measurements of plasma methoxytyramine, normetanephrine, and metanephrine as discriminators of different hereditary forms of pheochromocytoma. Clin Chem 2011;57:411-20. [Crossref] [PubMed]
- Fishbein L, Nathanson KL. Pheochromocytoma and paraganglioma: understanding the complexities of the genetic background. Cancer Genet 2012;205:1-11. [Crossref] [PubMed]
- Mariani-Costantini R, editor. Paraganglioma: A Multidisciplinary Approach. Brisbane AU: 2019 Codon Publications.; 2019.
- Wachtel H, Kennedy EH, Zaheer S, et al. Preoperative Metyrosine Improves Cardiovascular Outcomes for Patients Undergoing Surgery for Pheochromocytoma and Paraganglioma. Ann Surg Oncol 2015;22:S646-S654. [Crossref] [PubMed]
- Steinsapir J, Carr AA, Prisant LM, et al. Metyrosine and pheochromocytoma. Arch Intern Med 1997;157:901. [Crossref] [PubMed]
- Engelman K, Horwitz D, Jequier E, Sjoerdsma A. Biochemical and pharmacologic effects of alpha-methyltyrosine in man. J Clin Investig 1968;47:577-94. [Crossref] [PubMed]
- Serri O, Comtois R, Bettez P, et al. Reduction in the size of a pheochromocytoma pulmonary metastasis by metyrosine therapy. N Engl J Med 1984;310:1264-5. [Crossref] [PubMed]
- Spector S, Sjoerdsma A, Udenfriend S. Blockade of endogenous norepinephrine synthesis by α-methyl-tyrosine, an inhibitor of tyrosine hydroxylase. J Pharmacol Exp Ther 1965;147:86-95. [PubMed]
- Robinson RG, Dequattro V, Grushkin CM, et al. Childhood pheochromocytoma: treatment with alpha methyl tyrosine for resistant hypertension. J Pediatr 1977;91:143-7. [Crossref] [PubMed]
- Pyörälä K, Pitkänen E, Toivonen S. Alpha-methyl-p-tyrosine in the symptomatic treatment of patients with malignant phaeochromocytoma. Ann Med Intern Fenn 1968;57:65. [PubMed]
- Begnall WE, Salway JG, Jackson EW. Phaeochromocytoma with myocarditis managed with alpha-methyl-p-tyrosine. Postgrad Med J 1976;52:653-6. [Crossref] [PubMed]
- Amery A, Moerman EJ, Bossaert H, et al. α-methyl-p-tyrosine in malignant pheochromocytoma. Pharmacol Clin 1969;1:174-6. [Crossref]
- DEMSER (metyrosine) capsule. FDA prescribing information. Available online: https://www.drugs.com/pro/demser.html. Accessed October 31 2016.
- ICH harmonized tripartite guideline: guideline for good clinical practice E6 (R1). Current step 4 version 1996. Available online: https://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Efficacy/E6/E6_R1_Guideline.pdf. Accessed June 15 2017.
- Okun R. New medications-1981. Annu Rev Pharmacol Toxicol 1981;21:597-604. [Crossref] [PubMed]
- Delaney JP, Paritzky AZ. Necrosis of a pheochromocytoma with shock. N Engl J Med 1969;280:1394. [Crossref] [PubMed]
- Atuk NO, Teja K, Mondzelewski P, et al. Avasucular necrosis of pheochromocytoma followed by spontaneous remission. Arch Intern Med 1977;137:1073-5. [Crossref] [PubMed]
- van der Horst-Schrivers AN, Kerstens MN, Wolffenbuttel BH. Preoperative pharmacological management of phaeochromocytoma. Neth J Med 2006;64:290-5. [PubMed]
- Sanna M, Shin SH, Piazza P, et al. Infratemporal fossa approach type a with transcondylar-transtubercular extension for Fisch type C2 to C4 tympanojugular paragangliomas. Head Neck 2014;36:1581-8. [Crossref] [PubMed]
- Jackson CG. Glomus tympanicum and glomus jugulare tumors. Otolaryngol Clin North Am 2001;34:941-70. vii. [Crossref] [PubMed]
- Hernández-Guijo JM, Gandia L, Cuchillo-Ibanez I, et al. Altered regulation of calcium channels and exocytosis in single human pheochromocytoma cells. Pflugers Arch 2000;440:253-63. [Crossref] [PubMed]
- Isosaki M, Minami N, Nakashima T. Calphostin C, a potent and specific inhibitor of protein kinase C, reduces phorbol ester-induced but not primary Ca(2+)-induced catecholamine secretion from digitonin-permeabilized bovine adrenal medullary cells. Jpn J Pharmacol 1994;64:217-9. [Crossref] [PubMed]
- Knight DE, Baker PF. The phorbol ester TPA increases the affinity of exocytosis for calcium in 'leaky' adrenal medullary cells. FEBS Lett 1983;160:98-100. [Crossref] [PubMed]
- TerBush DR, Holz RW. Effects of phorbol esters, diglyceride, and cholinergic agonists on the subcellular distribution of protein kinase C in intact or digitonin-permeabilized adrenal chromaffin cells. J Biol Chem 1986;261:17099-106. [PubMed]
- Proye C, Thevenin D, Cecat P, et al. Exclusive use of calcium channel blockers in preoperative and intraoperative control of pheochromocytomas: hemodynamics and free catecholamine assays in ten consecutive patients. Surgery 1989;106:1149-54. [PubMed]
- Bravo EL. Pheochromocytoma. Cardiol Rev 2002;10:44-50. [Crossref] [PubMed]
- Lebuffe G, Dosseh ED, Tek G, et al. The effect of calcium channel blockers on outcome following the surgical treatment of phaeochromocytomas and paragangliomas. Anaesthesia 2005;60:439-44. [Crossref] [PubMed]
- Bravo EL. Pheochromocytoma: an approach to antihypertensive management. Ann N Y Acad Sci 2002;970:1-10. [Crossref] [PubMed]
- Bravo EL. Evolving concepts in the pathophysiology, diagnosis, and treatment of pheochromocytoma. Endocr Rev 1994;15:356-68. [Crossref] [PubMed]
- Malchoff CD, Macgillivray D, Shichman S. Pheochromocytoma Treatment. In: Mansoor GA (eds). Secondary Hypertension. Clinical Hypertension and Vascular Diseases. Humana Press, Totowa, NJ 2004.
- Lim KS, Low TC, Ng BK, et al. A case report of the use of magnesium sulphate during anaesthesia in a patient who had adrenalectomy for phaeochromocytoma. Ann Acad Med Singapore 2000;29:518-20. [PubMed]
Cite this article as: Liu Y, Wang Y, Zhou M, Zhang L, Pang Y, Wang C, Xiao Q, Liu L. Metabolism and secretion mechanism of catecholamine syndrome and related treatment strategies. J Xiangya Med 2020;5:39.


