
Video-assisted thoracoscopy in the diagnosis and treatment of malignant pleural disease
Introduction
The malignant pleural effusion is frequently connected with metastatic tumors that have arisen in other body areas as well as the primary tumor of the pleura. Besides the direct diffusion, cancer cells spread by hematic or lymphatic way is the pathogenic mechanism responsible of the malignant pleural effusion. Lung cancer is the main cause of malignant pleural effusion with a prevalence of 35%; the second most frequent cancer correlated with malignant pleural effusion is breast cancer. Nevertheless, up to 7% of malignant pleural effusions have an unknown origin (1). The actual incidence of malignant pleural effusion is somewhat uncertain, but it is estimated that over 150,000 new cases are diagnosed annually in the United States (2). Video-assisted thoracic surgery (VATS) biopsy and pleurodesis are widely used but there is no uniform approach to their implementation (3).
This narrative review focuses on the VATS diagnostic approach and treatment modalities for management of malignant pleural effusion.
Methods
We conducted a literature search through The National Library of Medicine database Medline, Embase and Cochrane Library by the following search terms: (Pleural Effusion, Malignant) AND (((VATS) OR Thoracic Surgery, Video-Assisted) OR “local anesthetic thoracoscopy”).The search was limited to English language papers published in the last 10 years; clinical case reports were excluded. Search results were downloaded; abstracts and full text articles were obtained to identify potentially relevant study. Inclusion criteria were randomized trials as well as prospective and retrospective cohort studies. Exclusion criteria were review papers and editorials.
A second search was made in the same database with search terms for Pleural effusion. The search was limited to English language guideline published in the last 10 years. A manual search was also undertaken.
Results
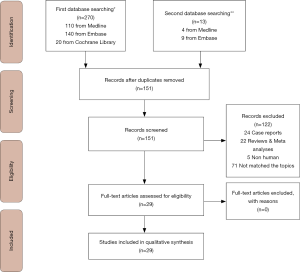
Of 151 records screened by the first search, 25 papers met the inclusion criteria; five, nine and eleven papers were randomized trials (4-8), prospective (9-17) and retrospective studies (18-28), respectively. The second search identified three guidelines (29-31) and a European survey (32). The Figure 1 shows the PRISMA flow diagram.
Discussion
Although the malignant pleural effusion engages the thoracic surgeon rather frequently in his daily practice, the literature is not particularly rich in scientific articles or guidelines.
The British guideline for investigation of a unilateral pleural effusion in adults recommended the pleural aspiration as the first diagnostic approach (30). The probability of getting the diagnosis from thoracentesis depends on the type of malignancy affecting the pleura: the sensitivity of the cytological examination is relatively high for adenocarcinomas (83–57%) while it is very limited for pleural mesotheliomas (20%) (33). It follows that many suspected malignant pleural effusions remain undiagnosed after cytological examination of the pleural fluid.
Tumor molecular profiling, especially for lung adenocarcinoma, has become essential in predicting the response to targeted therapy. Recent trial evaluated the diagnostic performance of new generation sequencing to achieve oncogene analysis on pleural effusion with encouraging results (34), but surgical biopsy guarantees better diagnostic adequacy given the greater amount of neoplastic tissue available for molecular tests (35).
Advanced genome research showed that metastases exhibit specific characteristics that are different from their primary tumor (36). Genomic, epigenetic, and other molecular changes found primarily in metastases could affect therapeutic strategy; thus, oncologists increasingly require biopsy of metastases from already known primary tumors.
Considering that up to 40% of cytological examinations of pleural effusions are inadequate and that tissue specimens suitable for molecular tests are increasing requested, pleural biopsy by endoscopic procedure is the preferred diagnostic tool for malignant pleural effusion in patients who can tolerate simple surgical manoeuvres as well as subsequent oncological therapy (29,32).
Pleuroscopy
Pleuroscopy, or medical thoracoscopy, is a procedure introduced by Jacobeus the early 1900s. Interventional pulmonologists manage rigid or semirigid pleuroscopes equipped with biopsy forceps while patients are under moderate sedation, breathing spontaneously. Recently, Canadian researchers published a retrospective study aimed to compare the diagnostic value of pleuroscopy and VATS pleural biopsy (19). The VATS procedures were performed under general anesthesia and single lung ventilation. Diagnostic adequacy was 93.5% and 96% for pleuroscopy and VATS biopsy, respectively. The authors concluded that pleuroscopy and VATS had similar diagnostic adequacy and safety characteristics. However, being pleuroscopy cheaper than VATS, the authors conclude that the former can be considered the technique of choice in selected settings.
VATS
VATS can be considered the surgical version of medical thoracoscopy. Over the past two decades, thoracic surgeons have successfully developed complex operations by VATS. Thoracic surgeons consider pleural biopsy as the simplest procedures that can be performed by VATS; therefore, pleural biopsy is often the first surgical procedure performed by trainees.
A sort of synthesis between pleuroscopy and VATS emerged in recent years: non-intubated or awake VATS. This is a technique implemented to minimize the drawbacks related to tracheal intubation and single lung ventilation. Although this technique also applies to major lung surgery, its use in the management of malignant pleural effusion seems attractive (23). A recent meta-analysis pooling 27 studies found that non-intubated VATS produces less inflammatory responses and immune stimulation than classical intubated VATS, resulting in patients accelerated recovery and cost saving (37).
From a technical point of view, the number and quality of access to the thoracic cavity can affect the postoperative course. A small but interesting randomized trial analyzed three different types of trocar/wound-protector without highlighting any difference between the three devices (5). A recent Bulgarian trial prospectively analyzed the impact of the number of chest ports on pain and satisfaction of patients with malignant pleural effusion undergoing VATS (9). The authors concluded that one-port shows better results than the three-port VATS. It should be noted that this point is one of the most discussed among thoracic surgeons and a final decision is still far from being reached.
Sometime, the pleura of patients with suspected malignant pleural effusion is apparently normal. In these cases, help in identifying possible metastatic locations is essential; to meet this need, some researchers tested fluorescence techniques. Russian researchers positively tested fluorescence identification of pleural malignancy with 5-aminolaevulinic acid, whereas autofluorescence seems identify pleural metastases better than primary tumor of the pleura (17,25).
Talc pleurodesis
Bethune first described the use of “iodized” talc in the 1930s; his purpose was to create pleural adhesion “as preliminary to lobectomy”.
For pleurodesis purposes, talc is administered by thoracoscopy (talc poudrage) or in suspension through a chest tube (talc slurry). Given for sure that talc is the best sclerosing agent, it remains open to debate whether it is more effective talc poudrage or talc slurry (31). A British randomized trial, which was published in late 2019, examined the two methods of talc administration (38). Seventeen hospitals in the United Kingdom participated in the TAPPS (Evaluating the efficacy of Thoracoscopy And talc Poudrage versus Pleurodesis using talc Slurry) trial recruiting 330 patients; the pleurodesis failure rate was 22% and 24% in the poudrage group and in the slurry group, respectively. Although the authors acknowledge that the study was underpowered, they claimed that talc slurry compared with talc poudrage had no significant difference in pleurodesis failure rate at three months.
Alternative to talc pleurodesis
British researchers published the results of the MesoVATS trial in 2014 (6). That randomized trial was planned to find out whether VATS partial pleurectomy compared with talc pleurodesis improves survival, pleural effusion and quality of life in patients with pleural effusion secondary to malignant pleural mesothelioma. Unfortunately, changes in staging system, surgical technique and introduction of new chemotherapy occurred during the ten years of the study. Finally, the authors observed median overall survival of 13.1 and 13.5 months in the VATS group and talc pleurodesis group, respectively. On the other hand, quality of life was significantly better in the VATS group.
Recently, Greek researchers published a randomized trial aimed to compare survival after hyperthermic perfusion of the pleural cavity towards talc poudrage in patients with malignant pleural effusion and lung cancer (4). The study group (20 patients) received a pleural perfusion with carboplatin (500 mg/m2) at 41.5 °C for 45 minutes. The median survival was 8 and 9 months for hyperthermic and talc poudrage group, respectively.
Mohsen and collaborators published a randomized trial comparing the efficacy and safety of 10% povidone-iodine solution administered at bedside and VATS talc poudrage (8). The Authors concluded that the two treatments were equivalent; it should be noted that there are some concerns on the safety of intra-pleural administration of iodine solution.
The issue of best pleurodesis agent was the focus of a very recent Cochrane Library network meta-analysis (39). The Authors evaluated 55 papers reporting 21 different substances/procedures; talc poudrage resulted in higher pleurodesis success than bleomycin, tetracycline, mustine, interferon, indwelling pleural catheter, mitoxantrone and placebo. Finally, the Authors compared talc poudrage to talc slurry restricting analysis only to studies at low risk of bias; the two procedures had equivalent success rate.
Limitations
The current review has limitations; as every narrative review, it includes the risk of bias. We tried to mitigate this effect by reporting the results of recent systematic reviews. Our search strategy may not have identified all relevant clinical studies limiting the timeframe to the last decade. On the other hand, we were keen to review the most recent studies to highlight any news. Despite malignant pleura effusion is a common clinical situation, most studies contained a relatively small sample size, which can affect the reliability of the results. Finally, the current review is necessarily vague about the search for evidence, the relevance of included studies and their validity.
Conclusions
Malignant pleural effusion frequently affects the clinical evolution of patients with metastatic cancer. Correct diagnosis of pleural metastasis in low risk patients is mandatory in the era of target therapy even though the primary tumor is well known. Pleural biopsy remains the gold standard for the diagnosis of malignant pleural effusion. Pleural biopsy can be performed through pleuroscopy or VATS; the choice between the two methods lays on local hospital setting. A good compromise that combines the skill of the surgical approach with the advantages of avoiding general anesthesia is the non-intubated VATS. The type and number of trocars used during VATS appears to have no relevant impact on the short-term outcome. The best treatment to prevent the accumulation of pleural fluid is talc pleurodesis. If talc poudrage is administered during pleuroscopy or VATS, any effusion recurrence does not justify a new surgical approach given that talc slurry is equally effective.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Duilio Divisi, Roberto Crisci) for the series “Malignant Pleural Effusion” published in Journal of Xiangya Medicine. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jxym-20-52). The series “Malignant Pleural Effusion” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Awadallah SF, Bowling MR, Sharma N, et al. Malignant pleural effusion and cancer of unknown primary site: a review of literature. Ann Transl Med 2019;7:353. [Crossref] [PubMed]
- American Thoracic Society. Management of malignant pleural effusions. Am J Respir Crit Care Med 2000;162:1987-2001. [Crossref] [PubMed]
- Perikleous P, Waller DA. Video assisted thoracoscopic and open chest surgery in diagnosis and treatment of malignant pleural diseases. J Vis Surg 2017;3:85. [Crossref] [PubMed]
- Kleontas A, Sioga A, Pandria N, et al. Clinical factors affecting the survival of patients diagnosed with non-small cell lung cancer and metastatic malignant pleural effusion, treated with hyperthermic intrathoracic chemotherapy or chemical talc pleurodesis: a monocentric, prospective, randomized trial. J Thorac Dis 2019;11:1788-98. [Crossref] [PubMed]
- Dell'Amore A, Campisi A, Giunta D, et al. The influence of the trocar choice on post-operative acute pain after thoracoscopy. J Vis Surg 2018;4:104. [Crossref] [PubMed]
- Rintoul RC, Ritchie AJ, Edwards JG, et al. Efficacy and cost of video-assisted thoracoscopic partial pleurectomy versus talc pleurodesis in patients with malignant pleural mesothelioma (MesoVATS): an open-label, randomised, controlled trial. Lancet 2014;384:1118-27. [Crossref] [PubMed]
- Son HS, Lee SH, Darlong LM, et al. Is There a Role for a Needle Thoracoscopic Pleural Biopsy under Local Anesthesia for Pleural Effusions? Korean J Thorac Cardiovasc Surg 2014;47:124-8. [Crossref] [PubMed]
- Mohsen TA, Zeid AA, Meshref M, et al. Local iodine pleurodesis versus thoracoscopic talc insufflation in recurrent malignant pleural effusion: a prospective randomized control trial. Eur J Cardiothorac Surg 2011;40:282-6. [PubMed]
- Valchev D, Peeva K, Petrov D. Are postoperative pain and patient satisfaction influenced by the number of ports in VATS for malignant pleural effusion treatment? Postgrad Med 2020;132:62-5. [Crossref] [PubMed]
- Feng X, Zhu L, Xiong X, et al. Therapeutical effect of intrapleural perfusion with hyperthermic chemotherapy on malignant pleural effusion under video-assisted thoracoscopic surgery. Int J Hyperthermia 2018;34:479-85. [Crossref] [PubMed]
- Sakaguchi H, Ishida H, Nitanda H, et al. Pharmacokinetic evaluation of intrapleural perfusion with hyperthermic chemotherapy using cisplatin in patients with malignant pleural effusion. Lung Cancer 2017;104:70-4. [Crossref] [PubMed]
- Walker S, Zubrinic M, Massey C, et al. A prospective study of patient-centred outcomes in the management of malignant pleural effusions. Int J Palliat Nurs 2016;22:351-8. [Crossref] [PubMed]
- Kara M, Alzafer S, Okur E, et al. The use of single incision thoracoscopic pleurectomy in the management of malignant pleural effusion. Acta Chir Belg 2013;113:270-4. [Crossref] [PubMed]
- Alar T, Ozcelik C. Single-incision thoracoscopic surgery of pleural effusions for diagnosis and treatment. Surg Endosc 2013;27:4333-6. [Crossref] [PubMed]
- Basso SM, Mazza F, Marzano B, et al. Improved quality of life in patients with malignant pleural effusion following video-assisted thoracoscopic talc pleurodesis. Preliminary results. Anticancer Res 2012;32:5131-4. [PubMed]
- Hunt BM, Farivar AS, Vallières E, et al. Thoracoscopic talc versus tunneled pleural catheters for palliation of malignant pleural effusions. Ann Thorac Surg 2012;94:1053-7. [Crossref] [PubMed]
- Pikin O, Filonenko E, Mironenko D, et al. Fluorescence thoracoscopy in the detection of pleural malignancy. Eur J Cardiothorac Surg 2012;41:649-52. [Crossref] [PubMed]
- Dadaş E, Erdoğdu E, Toker A, et al. Effectiveness of Video-Assisted Thoracoscopic Surgery in Undiagnosed Exudative Pleural Effusions. Turk Thorac J 2019;20:188-91. [Crossref] [PubMed]
- McDonald CM, Pierre C, de Perrot M, et al. Efficacy and Cost of Awake Thoracoscopy and Video-Assisted Thoracoscopic Surgery in the Undiagnosed Pleural Effusion. Ann Thorac Surg 2018;106:361-7. [Crossref] [PubMed]
- Hu R, Jiang H, Li H, et al. Intrapleural perfusion thermo-chemotherapy for pleural effusion caused by lung carcinoma under VATS. J Thorac Dis 2017;9:1317-21. [Crossref] [PubMed]
- Yoon DW, Cho JH, Choi YS, et al. Predictors of survival in patients who underwent video-assisted thoracic surgery talc pleurodesis for malignant pleural effusion. Thorac Cancer 2016;7:393-8. [Crossref] [PubMed]
- Marchetti G, Valsecchi A, Indellicati D, et al. Ultrasound-guided medical thoracoscopy in the absence of pleural effusion. Chest 2015;147:1008-12. [Crossref] [PubMed]
- Mineo TC, Sellitri F, Tacconi F, et al. Quality of life and outcomes after nonintubated versus intubated video-thoracoscopic pleurodesis for malignant pleural effusion: comparison by a case-matched study. J Palliat Med 2014;17:761-8. [Crossref] [PubMed]
- Freeman RK, Ascioti AJ, Mahidhara RS. A propensity-matched comparison of pleurodesis or tunneled pleural catheter in patients undergoing diagnostic thoracoscopy for malignancy. Ann Thorac Surg 2013;96:259-63: discussion 263-4.
- Liman ST, Elicora A, Topcu S, et al. Value of autofluorescence in video-assisted thoracoscopic surgery in pleural diseases. Thorac Cardiovasc Surg 2013;61:350-6. [Crossref] [PubMed]
- Whitworth JM, Schneider KE, Fauci JM, et al. Outcomes of patients with gynecologic malignancies undergoing video-assisted thoracoscopic surgery (VATS) and pleurodesis for malignant pleural effusion. Gynecol Oncol 2012;125:646-8. [Crossref] [PubMed]
- Sayir F, Cobanoglu U, Mergan D, et al. Video-assisted thoracoscopic surgery for malignant pleural effusions. Asian Pac J Cancer Prev 2011;12:415-8. [PubMed]
- Barbetakis N, Asteriou C, Papadopoulou F, et al. Early and late morbidity and mortality and life expectancy following thoracoscopic talc insufflation for control of malignant pleural effusions: a review of 400 cases. J Cardiothorac Surg 2010;5:27. [Crossref] [PubMed]
- Bibby AC, Dorn P, Psallidas I, et al. ERS/EACTS statement on the management of malignant pleural effusions. Eur J Cardiothorac Surg 2019;55:116-32. [Crossref] [PubMed]
- Hooper C, Lee YC, Maskell N. Investigation of a unilateral pleural effusion in adults: British Thoracic Society Pleural Disease Guideline 2010. Thorax 2010;65:ii4-ii17. [Crossref] [PubMed]
- Roberts ME, Neville E, Berrisford RG, et al. Management of a malignant pleural effusion: British Thoracic Society Pleural Disease Guideline 2010. Thorax 2010;65:ii32-ii40. [Crossref] [PubMed]
- Scarci M, Caruana E, Bertolaccini L, et al. Current practices in the management of malignant pleural effusions: a survey among members of the European Society of Thoracic Surgeons. Interact Cardiovasc Thorac Surg 2017;24:414-7. [PubMed]
- Rodríguez-Panadero F. Medical thoracoscopy. Respiration 2008;76:363-72. [Crossref] [PubMed]
- Liu L, Shao D, Deng Q, et al. Next generation sequencing-based molecular profiling of lung adenocarcinoma using pleural effusion specimens. J Thorac Dis 2018;10:2631-7. [Crossref] [PubMed]
- Cooke DT, Gandara DR, Goodwin NC, et al. Outcomes and efficacy of thoracic surgery biopsy for tumor molecular profiling in patients with advanced lung cancer. J Thorac Cardiovasc Surg 2014;148:36-40. [Crossref] [PubMed]
- Allgayer H, Leupold JH, Patil N. Defining the "Metastasome": Perspectives from the genome and molecular landscape in colorectal cancer for metastasis evolution and clinical consequences. Semin Cancer Biol 2020;60:1-13. [Crossref] [PubMed]
- Yu MG, Jing R, Mo YJ, et al. Non-intubated anesthesia in patients undergoing video-assisted thoracoscopic surgery: A systematic review and meta-analysis. PLoS One 2019;14:e0224737. [Crossref] [PubMed]
- Bhatnagar R, Piotrowska HEG, Laskawiec-Szkonter M, et al. Effect of Thoracoscopic Talc Poudrage vs Talc Slurry via Chest Tube on Pleurodesis Failure Rate Among Patients With Malignant Pleural Effusions: A Randomized Clinical Trial. JAMA 2019;323:60-9. [Crossref] [PubMed]
- Dipper A, Jones HE, Bhatnagar R, et al. Interventions for the management of malignant pleural effusions: a network meta-analysis. Cochrane Database Syst Rev 2020;4:CD010529. [PubMed]
Cite this article as: Nosotti M, Mazzucco A, Daffrè E, Cattaneo M, Ferrari M, Mendogni P. Video-assisted thoracoscopy in the diagnosis and treatment of malignant pleural disease. J Xiangya Med 2020;5:27.


