
Obstructive uropathy in a patient with primary myelofibrosis and non-IgM monoclonal gammopathy of undetermined significance—a case report
Introduction
Primary myelofibrosis (PMF) is one of myeloproliferative disorders caused by clonal proliferation of hematopoietic stem cells. It is uncommon in chronic myeloproliferative diseases with an estimated of the incidence of 1.5 per 100,000 population per year (1). Median OS was 69 months (95% CI, 61–76 months) in a multicenter retrospective study of 1,054 patients diagnosed with PMF (2). According to literature, non-IgM monoclonal gammopathy of undetermined significance (MGUS) has little influence on outcome and survival. Renal involvement in patients with PMF and non-IgM MGUS was less pronounced, but extramedullary hematopoiesis (EMH) may lead to obstructive uropathy and acute renal failure. Here we present an interesting case of 54-year-old male with obstructive uropathy, PMF and non-IgM MGUS. We present the following case in accordance with the CARE Guideline (available at http://dx.doi.org/10.21037/jxym-20-48).
Case presentation
A 54-year-old male farmer presented to us with a history of foamy urine for more than 3 years, anasarca over the last 5 months and daily urine output had reduced to 600 mL. And progressive fatigue, shortness of breath, numbness of hands and foot and abdominal discomfort were noticed during the last 5 months. There was no history of gross hematuria, urinary frequency and urgency, chest pain, fever, cough, etc. Other comorbidities included (I) splenohepatomegalia and cardiomegaly for unknown reasons for more than 20 years, the size of the spleen was 85×232 mm2 by ultrasound, echocardiography confirmed presence of cardiomegaly [left ventricle (LV): DM: 66 mm SM: 57 mm, right ventricle (RV): 30 mm, left atrium (LA): 43 mm, right atrium (RA): 33 mm)]; (II) gout for 10 years with the intermittent use of allopurinol and colchicine tablets. The patient received extracorporeal shock-wave lithotripsy (ESWL) for the treatment of urinary tract stone disease 10 years ago and cholecystectomy 4 years ago, and underwent a transfusion of 400 mL of concentrated red blood cells (CRBC) for severe anemia last year. No family member was suffering from similar illness.
On examination, fully oriented with pulse 87/min, BP =137/100 mmHg, RR =20 breaths/min and O2 saturation 98%. Ecchymosis were found on right lumber region. A few moist rales were heard at the bases of both lungs. The cardiac impulse was at the 5th intercostal space with 1cm. outside the left mid-clavicular line, with an enlarged area of cardiac dullness. Bilateral pedal edema was noted, the liver was palpable 1 cm below right costal margin, the spleen was 11 cm below left costal margin and +2 cm away from the midline, with predominant ascites. No lymphadenopathy was appreciated.
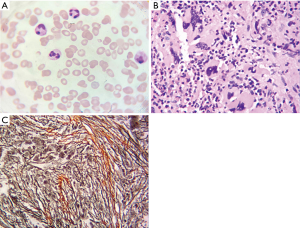
Hematological and biochemical tests on presentation (Table 1) suggest anemia, hyperkalemia, kidney injury associated with mild hyperphosphatemia and hyperuricemia and unusual thyroid hormones. Monoclonal proteins (M proteins) were measured and IgA M proteins were found in serum. Electroneuromyography showed peripheral nerve dysfunction. Trans-abdominal sonography confirmed splenohepatomegalia and left renal pelvis. PET-CT scan showed normal fluorodeoxyglucose (FDG) uptake, but displayed polyserositis including pericardium, pleural, celiac and pelvic cavities. Bone marrow cytology showed bone marrow hyperplasia was active with decreased erythroid series and increased granulocyte series (Figure 1A), bone marrow biopsy was suggestive of myelofibrosis (MF) with a few plasma cells (Figure 1B,C). In addition, V617F JAK2 and G543C DNMT3A mutation were found.
Table 1
| Parameter | Laboratory range | Values |
|---|---|---|
| Hemoglobin (g/dL) | 130–175 | 73.0 |
| TLC (109 cells/L) | 1.8–6.3 | 7.1 |
| Platelet count (109 cells/L) | 125–350 | 104.0 |
| RBC count (1012 cells/L) | 4.30–5.80 | 2.41 |
| MCV (fL) | 82–100 | 96.4 |
| MCHC (g/L) | 316–354 | 314.9 |
| Reticulocyte count (%) | 0.5–1.5 | 2.63 |
| ESR (mm/h) | 0–21 | 5.0 |
| PT (secs) | 10.0–16.0 | 14.1 |
| APPT (secs) | 25.0–43.0 | 46.1 |
| BUN (mmol/L) | 3.10–8.00 | 23.42 |
| S. Cr (μmol/L) | 41.0–111.0 | 271.8 |
| Uric Acid (μmol/L) | 208.0–428.0 | 589.0 |
| S. Sodium (mmol/L) | 137.0–147.0 | 143.1 |
| S. Potassium (mmol/L) | 3.50–5.30 | 5.43 |
| S. Chloride (mmol/L) | 99.0–110.0 | 112.7 |
| S. Calcium (mmol/L) | 2.00–2.60 | 1.95 |
| Phosphorus (mmol/L) | 0.86–1.78 | 1.23 |
| Total bilirubin (μmol/L) | 1.7–17.1 | 23.3 |
| Direct bilirubin (μmol/L) | 0.0–6.8 | 7.5 |
| S. Alkaline phosphatase (U/L) | 45.0–125.0 | 74.1 |
| LDH (U/L) | 120.0–250.0 | 332.9 |
| S. Albumin (g/L) | 40.0–55.0 | 30.2 |
| FT3 (pmol/L) | 2.8–7.1 | 1.63 |
| FT4 (pmol/L) | 12–22 | 7.33 |
| TSH (mIU/L) | 0.27–4.2 | 69.18 |
| TNF-α (pg/mL) | <8.1 | 37.1 |
| Urine analysis | PH 5.5, protein: +, low molecular weight portion:84.94% |
TLC, total leucocyte count; MCV, mean corpuscular volume; MCHC, mean corpuscular hemoglobin concentration; ESR, erythrocyte sedimentation rate; PT, prothrombin time; APTT, activated partial thromboplastin time; BUN, blood urea nitrogen; S. Cr, serum creatinine; LDH, lactate dehydrogenase; FT3, free triiodothyronine; FT4, free thyroxine; TSH, thyroid stimulating hormone; TNF-α, tumor necrosis factor.
Discussion
PMF has no specific clinical manifestations, including fatigue, symptoms due to an enlarged spleen, weight loss and others signs of a hypermetabolic state. Hematological and biochemical tests were also nonspecific, such as anemia, splenomegaly, either high or low white blood cell and platelet (3). In this report, the patient was admitted to our department because of foamy urine and anasarca, with decreased urine output, progressive fatigue, shortness of breath, numbness of hands and foot and abdominal discomfort. Combine his symptoms with electroneuromyography result, splenohepatomegalia, unusual thyroid hormones and IgA M proteins, POEMS syndrome came to our mind in the beginning, but hematologist suggested us to perform bone marrow examinations to confirm diagnosis. Bone marrow cytology was not diagnostic, while bone marrow biopsy showed replacement of the marrow by fibrosis with V617F JAK2 and G543C DNMT3A mutation. So, with those results and clinical features, we draw the conclusion that PMF and non-IgM MGUS were the final diagnosis, which were made according to standards supplied by WHO in 2016 (4) and International Myeloma Working Group in 2014 (5). This patient was diagnosed with hypothyroidism according to the disorder of thyroid hormone, while there in no research to indicate PMF may affect thyroid hormone levels, so we’re not sure if there’s a connections between PMF and hypothyroidism in this patient.
Bone marrow fibrosis and EMH are important features in PMF. EMH can be involved in any organ, but it is rare to find renal involvement. Hyperuricemic renal disease was reported, spontaneous tumor lysis syndrome (TLS) was presented in an advanced PMF patient that resolved with renal replacement therapy and uric-acid-lowering drugs (6). Ganguli et al. reported a case of obstructive uropathy and acute kidney injury as an initial presentation of PMF (7). This patient was displaying splenohepatomegalia for more than 20 years, gout for 10 years, and ESWL for the treatment of urinary stone means renal function has been influenced by PMF more than 10 years ago. Therefore, we present a rare case of obstructive uropathy with PMF and non-IgM MGUS.
There are some case reports and reviews reporting patients with coexisting PMF and MGUS (8). No evidence for these 2 entities arises from a same ancestor hematopoietic stem cell. The occurrence of M protein in myeloproliferative neoplasm (MPN) did not differ significantly from that observed in others. The medium survival of patients with PMF and MGUS is only slightly shorter than patients only with PMF. Approach to managing patients with coexistent MGUS and MPN is to treat the MPN and monitor for MGUS progression and potential complications. So, management strategies are aimed at treating the PMF and monitoring the non-IgM MGUS for transformation to an overt plasma-cell malignancy for this patient.
Hematopoietic cell transplantation (HCT) is the only possible way to obtain a cure for patients with PMF. Allogenic HCT is an effective treatment to lower the death rate and prolong survival for those patients. Relief of symptoms and improve the quality of life are the most important goals for all patients with PMF (9). For patients who are eligible for allogeneic HCT, they are often suggested transplantation rather than symptom-directed therapy, but the transplant-related mortality rate is high. Efficacy of allogeneic HCT depends on the chemotherapy and the graft-versus-tumor (GVT) effect from immune system. And allogeneic HCT could cause graft-versus-host disease (GVHD). Make a decision for transplantation must take into account many factors. In consideration of physiologic condition, laboratory tests and financial status, hematologist thought allogeneic HCT was not the best choice for this patient and recommended ruxolitinib because of V617F JAK2 mutation. Ruxolitinib 10 mg twice daily was used in view of impaired renal function. The most common mutated gene is JAK2 in patients with PMF. Ruxolitinib is the next best choice of treatment for PMF patients, a JAK-2 kinase inhibitor, which can provide symptomatic relief but not prolong survival.
Dynamic international prognostic scoring system (DIPSS) is a useful prognostic tool in patients with PMF, and also effectively stratifies for progression to leukemia (10). We identified our patient in high risk group in view of hemoglobin concentration and constitutional symptoms, the estimate medium OS is just 1.5 years (11) and at a higher risk for progression to leukemia (10). Luckily, medium OS was 5.9 years for PMF patients with a mutation of V617F JAK2 in a retrospective study (12), while another multivariate analysis reported 2.2 years (13). G543C DNMT3A mutation suggests in the development of the acute myeloid leukemia (14).
In summary, we report a rare case of goat, obstructive uropathy and renal failure with PMF and non-IgM MGUS, the patient has started treatment with ruxolitinib, we will follow-up changes in patient´s condition.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at http://dx.doi.org/10.21037/jxym-20-48
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jxym-20-48). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this report and any accompanying images.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Mesa RA, Silverstein MN, Jacobsen SJ, et al. Population-based incidence and survival figures in essential thrombocythemia and agnogenic myeloid metaplasia: An Olmsted county study, 1976-1995. Am J Hematol 1999;61:10-5. [Crossref] [PubMed]
- Cervantes F, Dupriez B, Pereira A, et al. New prognostic scoring system for primary myelofibrosis based on a study of the International Working Group for Myelofibrosis Research and Treatment. Blood 2009;113:2895-901. [Crossref] [PubMed]
- Tefferi A, Lasho TL, Jimma T, et al. One thousand patients with primary myelofibrosis: the mayo clinic experience. Mayo Clin Proc 2012;87:25-33. [Crossref] [PubMed]
- Arber DA, Orazi A, Hasserjian R, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood 2016;127:2391-405. [Crossref] [PubMed]
- Rajkumar SV, Dimopoulos MA, Palumbo A, et al. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol 2014;15:e538-48. [Crossref] [PubMed]
- Sile S, Wall BM. Acute renal failure secondary to spontaneous acute tumor lysis syndrome in myelofibrosis. Am J Kidney Dis 2001;38:E21. [Crossref] [PubMed]
- Ganguli A, Chalokia RS, Kaur BJ. Obstructive Uropathy as an Initial Presentation of Primary Myelofibrosis: Case Report and Review of Literature. Indian J Hematol Blood Transfus 2016;32:117-20. [Crossref] [PubMed]
- Malhotra J, Kremyanskaya M, Schorr E, et al. Coexistence of myeloproliferative neoplasm and plasma-cell dyscrasia. Clin Lymphoma Myeloma Leuk 2014;14:31-6. [Crossref] [PubMed]
- Cervantes F. Modern management of myelofibrosis. Br J Haematol 2005;128:583-92. [Crossref] [PubMed]
- Passamonti F, Cervantes F, Vannucchi AM, et al. Dynamic International Prognostic Scoring System (DIPSS) predicts progression to acute myeloid leukemia in primary myelofibrosis. Blood 2010;116:2857-8. [Crossref] [PubMed]
- Passamonti F, Cervantes F, Vannucchi AM, et al. A dynamic prognostic model to predict survival in primary myelofibrosis: a study by the IWG-MRT (International Working Group for Myeloproliferative Neoplasms Research and Treatment). Blood 2010;115:1703-8. [Crossref] [PubMed]
- Tefferi A, Guglielmelli P, Larson DR, et al. Long-term survival and blast transformation in molecularly annotated essential thrombocythemia, polycythemia vera, and myelofibrosis. Blood 2014;124:2507-615. [Crossref] [PubMed]
- Tefferi A, Guglielmelli P, Nicolosi M, et al. GIPSS: genetically inspired prognostic scoring system for primary myelofibrosis. Leukemia 2018;32:1631-42. [Crossref] [PubMed]
- Fried I, Bodner C, Pichler MM, et al. Frequency, onset and clinical impact of somatic DNMT3A mutations in therapy-related and secondary acute myeloid leukemia. Haematologica 2012;97:246-50. [Crossref] [PubMed]
Cite this article as: Quan J, Xu H, Wu D, Luo Y, Tao L, Peng Z. Obstructive uropathy in a patient with primary myelofibrosis and non-IgM monoclonal gammopathy of undetermined significance—a case report. J Xiangya Med 2020;5:18.


