
Multifocal adult Langerhans cell histiocytosis with diabetes insipidus as the initial symptom: a case report and literature review
Langerhans cell histiocytosis (LCH) is a rare disease with an annual incidence of 0.5–5.4 per million. The disease is divided into three clinical syndromes including Letterer-Siwe disease (LS), Hand-Schüller-Christian disease (HSC), and eosinophilic granuloma of bone (EGB). LCH has complicated clinical manifestations and mainly affects children aged 1–3 years (1-3), previous studies on its clinical features and treatments were also mainly conducted in children. Early diagnosis of LCH is difficult due to its insidious onset. Here we present an adult case, whose initial symptom was central diabetes insipidus (CDI), and gradually progressed to multifocal and multisystemic eosinophilic granuloma. We summarize the clinical features, diagnosis, radiation, pathology, immunohistochemically characteristics of this patient, along with literature review. We present the following case in accordance with the CARE reporting checklist (available at http://dx.doi.org/10.21037/jxym-20-45).
Case presentation
Written informed consent was obtained from the patient for publication of this study and any accompanying images.
A 32-year-old woman was admitted to our hospital due to “CDI for 8 years and left hip pain for 2 months” in November 2018. She developed polyuria, thirsty and polydipsia in 2010, was diagnosed as CDI and orally administered with desmopressin 0.1 mg tid in 2011. She suffered from lower back pain in 2010, and underwent a spine surgery in 2011, the pathology showed chronic inflammation of fibrous tissue. After the surgery, the low back pain was gradually improved and she began to suffer from pain at the left clavicle in 2017, then she developed left hip pain without any obvious cause with limited activity and a certain degree of lameness in September 2018. She was diagnosed of "hypothyroidism" in 2015 (TSH 2.18 µIU/mL, FT3 3.01 pmol/L, FT4 7.37 pmol/L, and was treated with replacement dose of levothyroxine sodium tablets (37.5 µg qd). Amenorrhea occurred when she was 25-year-old (2011), and oral medication was ap-plied to establish an artificial cycle. There was no family history of a similar disorder or a genetic disease.
Physical examination showed a mass sized about 2 cm × 3 cm was seen one third of the left clavicle, it was painful when pressed. The local skin was not ruptured, red, or swollen, although its temperature was slightly higher than that of contralateral side. Patrick sign was positive (+): the adduction was limited.
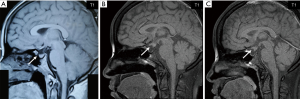
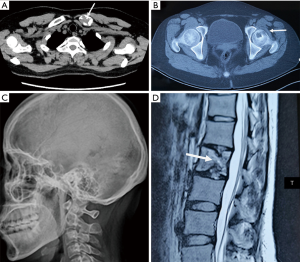
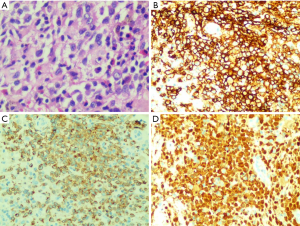
The laboratory results showed free light chains in blood and urine, immunoglobulins and complements, anti-GBM, anti-MPO, anti-PR3, anti-ANA, anti-dsDNA, anti-CCP antibodies, pANCA/cANCA, β-HCG, serum ACTH, cortisol levels and cortisol circadian rhythm were normal. Thyroid function: TSH3.5 µIU/mL, FT3 3.32 pmol/L, FT4 13.36 pmol/L, TPO-Ab >600 IU/mL, TG-Ab 262.8 IU/mL, TRAb 0.77 IU/mL (replacement of levothyroxine sodium tablets 37.5 µg qd), thyroid color ultrasound: diffuse enlargement of thyroid parenchyma, multiple thyroid parenchymal nodules: TI-RADS3. The sex hormone levels were as follows: FSH, 1.96 IU/L; LH, 1.75 IU/L; E, 254.25 pmol/L, P, 0.23 ng/mL; T, 0.356 ng/mL; PRL, 34.59 ng/mL .Whole-body single-photon emission computerized tomography (SPECT) bone scan revealed disorders of bone metabolism at multiple sites including left humerus, ribs, spine, and upper left femur. Magnetic resonance imaging (MRI) of the sellar region showed the disappearance of the high MR signal of the posterior pituitary gland, abnormal nodular thickening of the pituitary stalk and the anterior lobe of the pituitary is flat and the vacuole of the sella may be (Figure 1). X-ray of skull, limb long bones, and vertebrae bones showed that the bone density of some parts of the parietal bone decreased, along with the thinning of cortical bone; several sacciform bone density reduction areas near the sternal end of the left clavicle were visible; the bilateral L4 pedicles were abnormal; change in left sacroiliac joint was observed; suspicious decrease in local bone density was found in the left femoral head (Figure 2). Pathology confirmed the diagnosis of LCH in left clavicle, with the immunohistochemical findings including (Figure 3): CD1a (++), Ki67 (+; about 30%), S-100 (++), Langerin (++), and CD68 (+). The patient was diagnosed as LCH, she refused chemotherapy and was prescribed prednisone (30 mg/d) and nonsteroidal anti-inflammatory drugs (Mobic 7.5 mg/d). At the 1-month follow-up, the left hip pain was significantly relieved and she could walked normally, and she stopped Mobic 2 months later. The symptoms of CDI did not change after treatments, there was no obvious changes in MRI pituitary stalk at the 5-month follow-up (Figure 1C), and she was still orally administered with desmopressin 0.1 mg tid, the total amount of urine is about 1,500–2,000 mL per day, the urine specific gravity was 1.010 and urine osmotic pressure was 460 mOsm/L. After 6 months, the patient's condition was stable, with no new pain or discomfort.
Discussion
LCH is a disease characterized by the proliferation of mononuclear macrophage and dendritic cell systems. The etiology of the disease is not clear now, and it is generally believed to be related to the reactive lesions, abnormal clone proliferation, immune disorders, viral infection and other factors. Traditionally LCH is divided into four types (4): Type I: LS disease, with fever, rash, hepatosplenomegaly, and external ear pus as its main manifestations, which may be accompanied by head and neck masses in some cases; the patients may also develop hematological disorders (e.g., elevated peripheral blood leukocytes, anemia, and thrombocytopenia), liver dysfunction, bone and lung interstitial injuries, and involvement of multiple systems. Type II is an intermediate type between type I and type III. Type III, i.e., Hand–Schuller–Christian disease (HSC), is mainly manifested by exophthalmos, diabetes insipidus, and bone damage. Type IV, i.e., EGB, is often presented as local granuloma, with bone damage and pulmonary interstitial invasion as its main features, and the systemic involvement is mild. Due to the complicated clinical manifestations and the lack of a national registry, the exact prevalence of LCH in China remains unclear.
According to relevant guidelines, the diagnosis of LCH mainly relies on histopathological biopsy, during which typical Langerhans cells can be seen under light microscope, whereas the intracellular Birbeck granules visualized by electron microscopy are the “gold standard” for the diagnosis (5). The guidelines published in 2009 also indicated that a positive immunohistochemical finding of CD1a or Langerin also supports the diagnosis of LCH. For our case, the immunohistochemical results of biopsy tissue from left clavicle showed Langerin (++), confirmed the diagnosis of LCH.
A thorough literature review indicated that pituitary stalk thickening (by more than 3 mm) and disappearance of high-intensity signals in posterior pituitary on MRI are features of CDI (6), which should be distinguished from renal diabetes insipidus by fluid deprivation test. Girschikofsky et al. (7) found 29.6% of LCH patients had CDI, whose clinical manifestations occurred before the diagnosis or several months or even decades after the diagnosis. In a Chinese literature, the incidence of CDI among LCH patients was only 5% (8), significantly lower than those in European reports. In our current case, LCH began as CDI and then gradually affected bone tissues at multiple sites, but unfortunately bone biopsy failed to confirm the diagnosis, and MRI of sellar region has not been reexamined until she was admitted in our hospital 8 years later. According to the Lavin-Osband classification, the disease has progressed to grade II, and the chance of early treatment has been missed. Therefore, how to identify such patients as early as possible is an urgent issue to be addressed.
There is currently no uniform treatment for LCH. Multiple bone tissues and pituitary have been involved in our patient and there is no indication for local radiotherapy (9). The first-line chemotherapy (vincristine + prednisone) and second-line chemotherapy (cytosine arabinoside + cladribine) programs have been recommended in the Langerhans Cell Histiocytosis Evaluation and Treatment Guideline released by the Histolocyte Society in April 2009. A retrospective study on bone lesions in adult LCH patients(10), the treatment failure or recurrence rate in the low-dose cytosine arabinoside group (21%) was significantly lower than those in the 2-chlorodeoxyadenosine group (59%) and vinblastine/prednisone group (84%), along with lower incidences of toxicities, indicating low-dose cytosine arabinoside may be a more effective treatment for adult LCH patients.
In conclusion, we described a rare adult multifocal LCH case admitted as CDI in the department of Endocrinology. This adult multifocal LCH case showed the diversity of LCH onset symptoms, and it could start with a clinical symptom of any system, and then multiple system involved successively. The patient could be admitted in any department for treatment, and it is a big challenge for clinicians to diagnosis and treat the LCH timely. For the patients with pituitary stalk thickening, bone tissue and multiple organ involvement, the possibility of LCH should be considered and histopathological biopsy should be carried out, which is very important for the diagnosis and treatment.
Acknowledgments
Funding: This work was supported by the National Natural Science Foundation (Grant No.81873643) and Foundation of Hunan Provincial Science & Technology Department (Grant No. 2018ZK4033, 2019JJ40517), China.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at http://dx.doi.org/10.21037/jxym-20-45
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jxym-20-45). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this study and any accompanying images.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Steven H, Nancy LH, Stefano AP, et al. WHO Classification of Tumors of Haematopoietic and Lymphoid Tissues: Lyon: IARC press, 2008:358-360.
- Alston RD, Tatevossian RG, McNally RJ, et al. Incidence and survival of childhood Langerhans cell histiocytosis in Northwest England from 1954 to 1998. Pediatr Blood Cancer 2007;48:555-60. [Crossref] [PubMed]
- A multicentre retrospective survey of Langerhans' cell histiocytosis: 348 cases observed between 1983 and 1993. The French Langerhans' Cell Histiocytosis Study Group. Arch Dis Child 1996;75:17-24. [Crossref] [PubMed]
- Zhang ZN, Liu EK, Li XM, et al. Blood diagnosis and response criteria. Tianjin: Science and Technology Publishing House, 1990.
- Haupt R, Minkov M, Astigarraga I, et al. Langerhans cell histiocytosis (LCH): guidelines for diagnosis, clinical work-up, and treatment for patients till the age of 18 years. Pediatr Blood Cancer 2013;60:175-84. [Crossref] [PubMed]
- Zhang DK, Zhang JM. Langerhans cell histiocytosis in central nervous system. Journal of International Neurology and Neurosurgery 2007;34:61-4.
- Girschikofsky M, Arico M, Castillo D, et al. Management of adult patients with Langerhans cell histiocytosis: recommendations from an expert panel on behalf of Euro-Histio-Net. Orphanet J Rare Dis 2013;8:72. [Crossref] [PubMed]
- Xu X, Nie X, Xiong W, et al. A Clinicopathological Analysis of 160 Cases of Adult Langerhans Cell Histiocytosis. Zhonghua Xue Ye Xue Za Zhi 2015;36:135-9. [PubMed]
- Duan MH, Han X, Li J, et al. Efficacy of Radiotherapy for Adult Patients With Langerhans Cell Histiocytosis. Zhonghua Xue Ye Xue Za Zhi 2013;34:482-4. [PubMed]
- Cantu MA, Lupo PJ, Bilgi M, et al. Optimal therapy for adults with Langerhans cell histiocytosis bone lesions. PLoS One 2012;7:e43257. [Crossref] [PubMed]
Cite this article as: Zhou M, Yin J, Liao L, Wang M. Multifocal adult Langerhans cell histiocytosis with diabetes insipidus as the initial symptom: a case report and literature review. J Xiangya Med 2020;5:17.


