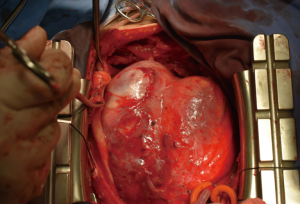
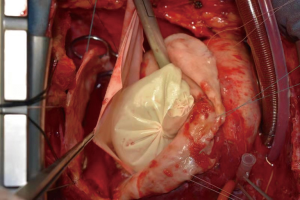
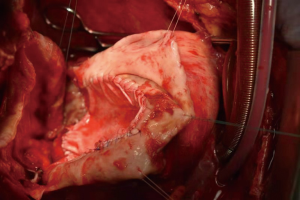
Ischemic mitral regurgitation combined with massive true ventricular aneurysm
Case presentation
Ventricular aneurysm is one of the common complications after transmural myocardial infarction, the incidence of which is about 10–35% (1). More than 95% of the true ventricular aneurysm originated from myocardial infarction, 85% of the true ventricular aneurysm was located in the anterolateral apical region, a few were located in the lateral wall, and only 5–10% were located in the posterior wall near the bottom of the apical base (2). Posterior inferior true ventricular aneurysm with mitral regurgitation, often secondary to papillary muscle ischemia, can lead to fatal heart failure (3).
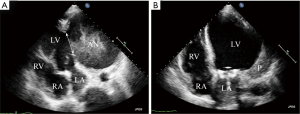
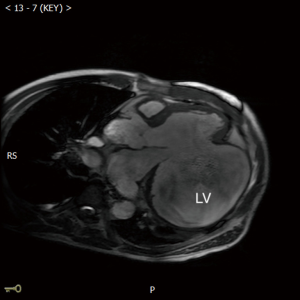
A 47-year-old man was admitted due to a 2-month history of chest tightness, and 20 kg of weight loss. Coronary angiography revealed 90% stenosis of the right coronary, occlusion of the circumflex artery. Electrocardiogram (ECG) showed a left bundle branch block, T-wave inversions in lead V4 to V6, and a heart rate of 88 beats/min. Ultrasound cardiograph (UCG) revealed aneurysms (120×110×100 mm3) in the inferior-posterior-lateral wall of the left ventricle and thrombus (Figure 1A); the area of the neck of the aneurysm was 60×70 mm3; the aneurysm volume was 640 mL; the aneurysm wall thickness was 3 mm; the left ventricular end diastolic diameter (LVEDD) was 146 mm; the left ventricular ejection fraction (LVEF) was 30%; and the area of mitral regurgitation was 7.0 cm2. The neck of aneurysm was repaired using a patch (Figure 1B). Cardiac magnetic resonance imaging (MRI) (Figure 2) showed that the aneurysm diameter was 96 mm and that the size of the aneurysm was 126×96 mm2. PET-CT showed that the LVEDV, left ventricular end systolic diameter (LVSDV), and LVEF was 234 mL, 165 mL, and 30%, respectively. The range of transmural myocardial infarction accounted for approximately 48% of the left ventricular area.
Coronary artery bypass grafting (AO-SVG-RCA, PI 1.2, flow 58 mL/min), mitral valve replacement (AP 26#), and left ventriculoplasty were performed with extracorporeal circulation. The key to the recovery of cardiac function is the suitable size of the patch for ventricular plasty. Therefore, a model made from a glove filled with saline was used to mark the suture line for the proposed repair area (Figures 3-5). The patient was extubated on postoperative day 8, and discharged on postoperative day 18. UCG before discharge showed LVEDD was 57 mm and LVEF was 44%, the area of the space between the pericardial patch and the residual aneurysm wall was 55×34 mm2.
At the 6-month follow-up visit, the patient had gained 10 kg. UCG showed LVEF and LVEDD of 47% and 62 mm; the diameter of the residual false lumen was 22 mm, and a thrombus was noted. MRI showed LVEDV, LVSDV, and cardiac output of 175 mL, 137 mL, and 2.93 L/min, respectively. Coronary CTA showed a patency of the vein graft.
Discussion
The key to the recovery of cardiac function after surgery is the size of the patch (4). Therefore, a model made from a glove filled with normal saline was used to mark the suture line for the proposed repair area. The disadvantages of this method are as follows. (I) The LVEDV in this patient was still higher than normal after surgery, possibly due to the custom model, which did not fit the left ventricle due to the irregular left ventricular chamber, causing the position of the proposed suture line to be higher than the ideal position. Therefore, the patch should be sutured to a site lower than the proposed suture line. (II) The patch should be designed to strength the aneurysm wall with the interrupted suture, which may reduce the effect of the paradoxical movement of the free wall on left ventricular systolic function and thereby improve cardiac function.
Acknowledgments
Funding: This work was supported by the National Natural Science Foundation of China (NSFC-81570373).
Footnote
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jxym.2019.10.01). The authors have no conflicts of interest to declare.
Ethical statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this Case report and any accompanying images.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Krähenbühl ES, Immer FF, Stalder M, et al. Technical advances improved outcome in patients undergoing surgery of the ascending aorta and/or aortic arch: ten years experience. Eur J Cardiothorac Surg 2008;34:595-9. [Crossref] [PubMed]
- Abrams DL, Edelist A, Luria MH, et al. Ventricular aneurysm. a reappraisal based on a study of sixty-five consecutive autopsied cases. Circulation 1963;27:164-9. [Crossref] [PubMed]
- Swan HJ. Left ventricular systolic and diastolic dysfunction in the acute phases of myocardial ischaemia and infarction, and in the later phases of recovery. Function follows morphology. Eur Heart J 1993;14 Suppl A:48-56.
- Henry MJ, Preventza O, Cooley DA, et al. Left ventricular aneurysm repair with use of a bovine pericardial patch. Tex Heart Inst J 2014;41:407-10. [Crossref]
Cite this article as: Li Y, Dong R. Ischemic mitral regurgitation combined with massive true ventricular aneurysm. J Xiangya Med 2020;5:9.