
Coexistence of multiple congenital anomalies: quadricuspid pulmonary valve, infundibular pulmonary stenosis, coronary artery anomaly, biventricular hypertrophy, and short stature
A 67-year-old man was admitted to our hospital because of worsening of exertional dyspnea over two years. The patient was short in stature: height, 147 cm (<2 standard deviations); his body weight was 43 kg. Abnormal facial features and thoracic deformities were absent. Jugular vein distension, hepatomegaly, and leg edema were not noted. A grade 3, low-pitched, systolic murmur was heard at the third intercostal space along the left sternal border. His blood pressure was 146/93 mmHg.
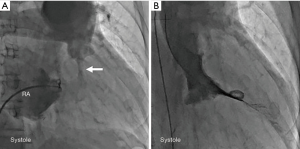
Electrocardiography revealed tall R waves and deep S waves in leads V1-5 and ST depression and T-wave inversion in leads V3-6, indicating biventricular hypertrophy (1). Chest radiography revealed a cardiothoracic ratio of 58%, prominence of the main pulmonary artery, and dilatation of the left pulmonary artery. No pleural effusion was detected. Transthoracic two-dimensional echocardiography revealed severe and concentric left ventricular hypertrophy: the septal wall thickness was 14 mm (normal: <11 mm) and the posterior wall thickness was 12 mm (normal: <11 mm); right ventricular hypertrophy: the basal wall thickness was 6 mm (normal: <4 mm); and moderate pericardial effusion (Figure 1A). Doppler echocardiography revealed an estimated peak pressure gradient of 69 mmHg between the right ventricle and the pulmonary artery. Three-dimensional-rendered computed tomography (CT) images revealed the absence of the circumflex coronary artery (Figure 1B, arrow) and high take-off of the right coronary: the right coronary artery ostium was located approximately 15 mm above the right sinus of Valsalva (Figure 1B), and aneurysmal dilatation of the main pulmonary artery was seen (Figure 1C). Contrast-enhanced cine CT demonstrated aneurysmal dilatation of the main pulmonary artery, measuring 45 mm (Figure 1D); pulmonary subvalvular narrowing (Figure 1D, arrow); and moderate pericardial effusion (Figure 1D, asterisks). Contrast-enhanced CT revealed a quadricuspid pulmonary valve (QCPV) with normal coaptation on closing during diastole and without restriction on opening during systole (Figure 1E). Cardiac catheterization revealed right ventricular hypertension and a systolic pressure gradient of 55 mmHg between the right ventricle and the pulmonary artery: right ventricular pressure of 93/12 mmHg and pulmonary artery pressure of 38/21 mmHg. His pulmonary capillary wedge pressure was 8 mmHg and cardiac output was 4.5 L/min. No intracardiac shunt was detected by blood oximetry sampling. Right atrial angiography revealed stenosis at the infundibulum of the right ventricle (Figure 2A, arrow) and hypertrophy of the right ventricle (Figure 2A), and left ventriculography revealed concentric hypertrophy (Figure 2B). Selective coronary angiography, as well as CT, revealed an absent left circumflex artery and a high take-off right coronary artery. No chromosomal abnormalities were detected by G-banding analysis. The patient did not provide consent for a gene analysis. Because the patient refused to undergo surgery, he was recommended to undergo beta-blocker therapy for ventricular hypertrophy associated with hyperdynamic systolic function during regular follow-up visits and has not shown worsening symptoms for 2 years.
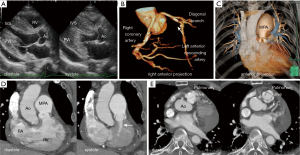
QCPV is a rare congenital anomaly in the general population (2), and infundibular pulmonary stenosis is also uncommon. In the present case, infundibular narrowing might have been caused by right ventricular fibromuscular hypertrophy below the pulmonary valve. Because no restrictions in pulmonary valve opening were observed on CT findings, aneurysmal dilatation of the main pulmonary trunk was explained as poststenotic dilatation caused by infundibular stenosis.
Coexistence of various cardiac malformations (QCPV, infundibular stenosis, coronary anomalies) and extra-cardiac anomalies (short stature) may be related to a genetic syndrome (3-5). Genetic syndromes encompass diverse clinical features including short stature; characteristic facial dysmorphism; thorax deformity; and cardiac anomalies, such as atrial septal defect, ventricular septal defect, aortic stenosis, bicuspid aortic valve, pulmonary stenosis, and hypertrophic cardiomyopathy (3). For example, the Noonan syndrome (RAS/MAPK syndrome) is associated with short stature, hypertelorism, pulmonary valve stenosis, and hypertrophic cardiomyopathy (4,6). On the other hand, hypertrophic cardiomyopathy can be complicated by pericardial effusion (7). In this case, hypertrophic cardiomyopathy presenting with biventricular hypertrophy associated with pericardial effusion may be a manifestation of a genetic syndrome. To our knowledge, the coexistence of five conditions, namely QCPV, infundibular pulmonary stenosis, coronary anomalies, biventricular hypertrophy, and short stature, has not been previously reported.
Acknowledgments
The authors thank Dr. Takeshi Yamashita, Dr. Wakana Takahashi, Dr. Saori Munemoto, Dr. Ryoichi Murata, Dr. Tsutomu Saga, and Dr. Mikio Ueda for their support and advice.
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jxym.2019.10.04). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Jain A, Chandna H, Silber EN, et al. Electrocardiographic patterns of patients with echocardiographically determined biventricular hypertrophy. J Electrocardiol 1999;32:269-73. [Crossref] [PubMed]
- Olivares-Reyes A, Molina-Bello E, Espinola-Zavaleta N. Congenital quadricuspid pulmonary valve in an adult patient with double valvular lesions and poststenotic dilatation of the trunk and the left branch of the pulmonary artery: a case presentation and review of the literature. Congenit Heart Dis 2012;7:E103-8. [Crossref] [PubMed]
- Ko JM. Genetic Syndromes associated with Congenital Heart Disease. Korean Circ J 2015;45:357-61. [Crossref] [PubMed]
- Gelb BD, Roberts AE, Tartaglia M. Cardiomyopathies in Noonan syndrome and the other RASopathies. Prog Pediatr Cardiol 2015;39:13-9. [Crossref] [PubMed]
- Thompson D, Patrick-Esteve J, Surcouf JW, et al. RAF1 variants causing biventricular hypertrophic cardiomyopathy in two preterm infants: further phenotypic delineation and review of literature. Clin Dysmorphol 2017;26:195-9. [Crossref] [PubMed]
- Formigari R, Michielon G, Digilio MC, et al. Genetic syndromes and congenital heart defects: how is surgical management affected? Eur J Cardiothorac Surg 2009;35:606-14. [Crossref] [PubMed]
- Fozing T, Zouri N, Adam O, et al. Management of a patient with pericardial decompression syndrome and HOCM. BMJ Case Rep 2016;2016:bcr2015211550. [Crossref] [PubMed]
Cite this article as: Mori K, Yagi M, Shimojima M, Maekawa N, Kuroda E, Takamura M, Yamagishi M. Coexistence of multiple congenital anomalies: quadricuspid pulmonary valve, infundibular pulmonary stenosis, coronary artery anomaly, biventricular hypertrophy, and short stature. J Xiangya Med 2020;5:8.


