Clinical report and multidisciplinary team discussion of two fatal cases of severe acute pancreatitis
Introduction
Severe acute pancreatitis (SAP) is the local inflammation of the pancreas that causes multiorgan dysfunction throughout the body. SAP can be caused by biliary tract disease, alcohol, hypertriglyceridemia, trauma, viral infection, pregnancy, etc., and has a case-fatality rate of up to 30–50%. Based on its disease course, SAP can be divided into early (within 7 days of disease onset) and late (after 7 days) stages. The main pathophysiological changes in the early stage are the deranged functions of circulatory, respiratory, and renal systems, and other organs primarily caused by the systemic inflammation, which may even lead to multiple organ dysfunction syndrome (MODS). In the late stage, SAP-related complications such as retroperitoneal infection, intra-abdominal hemorrhage, gastrointestinal fistula, and portal vein thrombosis can occur. With the advances in approaches to support organ functions, the case-fatality rate of SAP patients has dramatically decreased in the early stages, but unfortunately, the SAP-associated complications in the late stages have now become the main cause of death.
Herein, we report two patients who died of SAP-related complications in the late stage. Also, the inputs from a multidisciplinary team (MDT) of pancreaticobiliary experts are summarized.
Case presentation
Case 1
A 58-year-old male patient was admitted for “sudden onset of upper abdominal pain for one day” on January 7, 2017. One day before his admission, he suffered from sudden severe epigastric pain without any obvious cause, which radiated to his lower back, accompanied by nausea and vomiting. He also stopped passing flatus since onset, and later he developed icterus and jaundice. No chills or fever was noted but his urine output decreased significantly. About half a year ago, he was managed conservatively in a local hospital with a diagnosis of “cholecystitis”. Physical examinations showed: temperature (T) 37 °C; blood pressure (BP) 148/75 mmHg; respiratory rate (RR) 30/min; pulse rate (PR) 116/min. His skin and sclera were moderately yellow-stained. Pulmonary examination was normal. Abdominal distention, guarding, and abdominal tenderness were obvious, although no rebound tenderness was observed. Murphy sign was negative. His body mass index (BMI) was 25 kg/m2. The results of laboratory tests included the following: white blood cell (WBC) count, 20.4×109 /L; neutrophils (N), 91.5%; Hct, 51%; serum amylase, 1,131 U/L; TBIL, 233.3 µmmol/L; DBIL, 119.5 µmmol/L; ALT, 195.6 U/L; AST, 159.5 U/L; U-BIL, ++; URO, -; triglyceride, 3.2 mmol/L; BUN 15 mmol/L; creatinine, 211.4 µmol/L; Procalcitonin, 12.6 ng/ml; PCO2, 26 mmHg; PO2, 98 mmHg; and Lactate, 5.4 mmol/L.
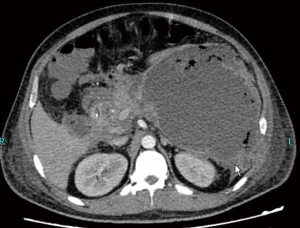
Ultrasound of the abdomen did not show any gallstone. Computed tomography (CT) scan revealed an enlarged pancreatic volume with exudative changes suggestive of acute pancreatitis (AP) (Figure 1). Patient was diagnosed with SAP. Aggressive fluid resuscitation was given within 24 hours of admission, followed by fluid resuscitation therapy. Patient was mechanically ventilated for respiratory failure for about 5 days from January 8 to 13. Hemodialysis therapy was performed from January 9 to 12; Initially, patient received prophylactic cefoperazone-sulbactam from January 7 to 17. He was started on enteral nutrition (EN) via nasogastric tube on January 12.
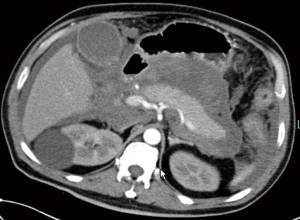
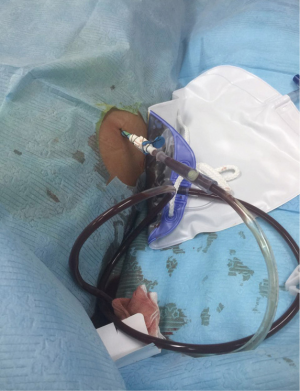
After the active treatments mentioned above, the patient's vital signs gradually stabilized. His fever and abdominal distension improved. Patient tolerated the tube feedings well. The liver transaminases gradually became normal. However, on January 17, the patient suddenly developed high fever of 40°C. He became hypotensive requiring vasopressors to maintain the circulation. His abdominal distension became obviously worse, and CT scan revealed “air bubble sign” inside the retroperitoneal space (Figure 2). Imipenem/cilastatin and tigecycline were given to treat the possible bacterial infection, and aggressive fluid resuscitation was given for shock. On January 18, a 16-F pigtail catheter was placed for drainage at bedside under ultrasound guidance (Figure 3). However, the abdominal distension did not improve. On January 19, the patient’s family members gave up the aggressive treatment and the patient was transferred to the local hospital where he later died.
Case 2
A 36-year-old male patient was admitted to the hospital due to “sudden onset of upper abdominal pain for 5 hours after a full meal” on June 20, 2016. Five hours before his admission, he suffered from sudden onset, sharp epigastric pain after eating greasy food, which was accompanied by nausea and vomiting. Patient also complaint of increasing difficulty of breathing since the onset of his symptoms. He also stopped passing flatus. In the past, he was repeatedly hospitalized for AP but recovered after conservative treatment. Physical examinations showed: T, 38.4 °C; BP, 107/75 mmHg; RR, 33/min; PR, 127/min. On physical exam, he was awake. Abdominal distention, guarding, and abdominal tenderness were obvious, although no rebound tenderness was observed. Cardiopulmonary examination was normal except for high heart rate of 127/min. His BMI was 35 kg/m2. The results of laboratory tests were: WBC 14.4×109 /L; N 83.5%; Hct 50%; Amylase 878 U/L; TBIL 16.3 µmmol/L; DBIL 7.5 µmmol/L; triglyceride 18.7 mmol/L; Procalcitonin 2.6 ng/mL; CRP, 231 mg/L; PCO2 29 mmHg; PO2 68 mmHg; and Lactate 4.4 mmol/L.
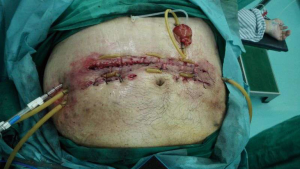
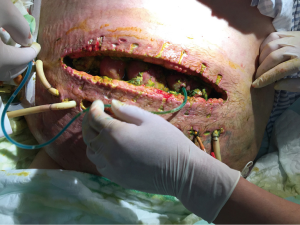
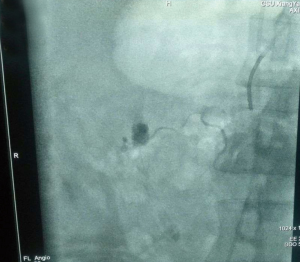
Extensive exudative changes were seen around the pancreas, and the shape of the pancreas was poorly defined on CT scan, which was consistent with AP. Diagnosis on admission was SAP (hyperlipidemic) with MODS. Patient was immediately started on mechanical ventilation and aggressive fluid resuscitation. He also underwent hemodialysis. No prophylactic antibiotics were used. EN therapy was started on the 5th day of disease onset. However, after a prolonged hospitalization, on July 19, patient developed sudden high fever and worsening of abdominal distension. A percutaneous catheter drainage (PCD) of wall-off necrosis (WON) was performed. Later, due to persistent fever and abdominal hemorrhage, he underwent open surgeries twice on July 26 and July 29. During these surgeries, pancreatic necrosectomy was performed. No obvious source of bleeding was confirmed. However, fever and intermittent intraabdominal bleeding persisted afterwards. He was then transferred to our hospital about 45 days after the onset on August 6. An intestinal fistula was discovered after uncovering the wound (Figure 4). On August 12, exploratory laparotomy with removal of peripancreatic necrotic tissue, jejunostomy, and abdominal drainage were performed under general anesthesia (Figure 5). However, two hours later patient again developed intraabdominal hemorrhage resulting in hypovolemic shock. Patient received blood transfusion. Digital subtraction angiography (DSA) revealed the rupture of a pseudoaneurysm in a branch of the superior mesenteric artery (Figure 6), which was successfully embolized. Unfortunately, on August 14, the patient died due to septic shock.
iMDT discussion
Discussion among physicians from Xiangya Hospital, Central South University, China
Diagnosis of SAP
Dr. Zhiyong Liu (Department of Critical Care Medicine):
The management of SAP includes laboratory evaluation, assessment of severity, and etiological diagnosis. According to the “Guidelines on the Diagnosis and Treatment of AP” (1) released by the Chinese Society of Pancreatic Surgery in 2014, two of the following three features are required for the diagnosis of AP: (I) abdominal pain consistent with AP; (II) serum lipase activity (or amylase activity) at least three times greater than the upper limit of normal; and (III) characteristic findings of AP on abdominal imaging. Both patients in our series presented with typical abdominal pain consistent with pancreatitis. The sharp knife-like pain was located in the upper abdomen and radiated to the lower back. The increase of serum amylase was more than three times of the normal value. In addition, abdominal CT in both patients revealed enlarged pancreatic volume and exudative changes around the pancreas. Both patients suffered from respiratory and renal failure which lasted more than 48 hours. According to the Revised Atlanta Classification of Acute Pancreatitis (2), a diagnosis of SAP was made. According to the literature (3), biliary tract diseases are the most common cause of AP, accounting for about 40% of cases. Hypertriglyceridemia is the third leading cause of AP after alcoholism, accounting for about 2–5% of causes. AP itself can lead to disorders of lipid metabolism and increase blood lipid levels. Therefore, hyperlipidemic AP can be considered when the serum triglyceride level is higher than 11.3 mmol/L. The triglyceride level in our second case reached 18.7 mmol/L, and a definite etiological diagnosis was made. The diagnosis of biliary AP is mainly based on abdominal imaging (gallstones or biliary sludges), and elevation in hepatic transaminases is also helpful. Endoscopic ultrasound (EUS) can also identify smaller gallstones or common bile duct (CBD) stones.
Dr. Junwen Yang (Department of Gastroenterology):
For patients with biliary AP combined with cholangitis or CBD obstruction, endoscopic retrograde cholangiopancreatography (ERCP) should be performed within 24 to 48 hours of presentation. In severe cases with unstable systemic conditions, percutaneous transhepatic gallbladder drainage may be considered if ERCP cannot be performed. In patients with suspected biliary pancreatitis, if there is no evidence of stones by ultrasound or CT, magnetic resonance cholangiopancreatography (MRCP) or EUS can be performed to improve the sensitivity in detecting biliary pancreatitis. In Case 1, a diagnosis of biliary SAP was made based on biochemical findings and past medical histories; however, CT did not identify any stones in the gallbladder or bile ducts. MRCP or EUS might be useful to find non-radio opaque stones.
Indicators for predicting progression of AP
Dr. Daomiao Xu (Department of Critical Care Medicine):
Patients with SAP are often treated in an intensive care unit (ICU) where the monitoring measures are more sophisticated (4). However, it is difficult for clinicians to identify patients who are more likely to develop SAP. Although there are many scoring systems (e.g., APACHE II, Marshall, and BISAP) for assessing disease severity, their abilities in predicting the progression of AP into a severe form remain unsatisfactory (5). Clinicians need to integrate some indicators in the early stage to predict whether such progression will occur. These indicators include the following: (I) the general characteristics of the patient, such as age >55 years, BMI >30 kg/m2, altered state of consciousness, and presence of multimorbid conditions; (II) the persistence of systemic inflammatory response syndrome (SIRS); (III) blood urea nitrogen (BUN) > 20 mg/dL, HCT >44%, and persistent increase in serum creatinine; and d) imaging changes, such as presence of pleural effusion and/or pulmonary exudate, and extensive or multiple fluid accumulation around the pancreas. In the early stage of the disease, both patients presented with persistent SIRS and HCT >44%, suggesting hemoconcentration. In addition, Case 2 had a BMI of up to 30 kg/m2. These indicators are highly predictive of a severe form of AP, and patients presenting with such indicators should be sent to the ICU earlier.
Early treatment of SAP
Dr. Lina Zhang (Department of Critical Care Medicine):
Patients with SAP suffer from insufficient circulatory volume due to the third space fluid loss because of increased capillary permeability due to profound inflammatory response. This issue gets even worse in patients with nil per os and severe vomiting. This leads to low perfusion of pancreatic tissue culminating into pancreatic necrosis. Therefore, early aggressive fluid resuscitation can help increase the effective circulatory volume, improving the pancreatic microcirculation, and thereby, reduce serious complications associated with pancreatic necrosis. Therefore, patients with SAP without contraindications such as congestive heart failure should undergo resuscitation with lactated Ringer’s (LR) solution at 250–500 mL/hr for at least 12–24 hours. However, volume overload can also increase the intra-abdominal pressure and aggravate acute respiratory distress syndrome (ARDS) associated with high morbidity and mortality (4). Thus, optimal hemodynamic management based on the appropriate assessment of volume responsiveness in SAP patients is particularly important. Traditional indicators such as BUN can be affected by impaired renal function and other underlying comorbid conditions; some other hemodynamic indicators such as central venous pressure (CVP) and mean arterial pressure (MAP) are also affected by increased intra-abdominal pressure. Finally, individual demographic differences can also affect these indicators in assessing the blood volume. Passive leg raising (PLR) is a simple method that is often used in the ICU to quickly determine the volume responsiveness of a patient at the bedside. However, the increased intra-abdominal pressure in SAP patients often decreases the sensitivity of PLR in predicting volume responsiveness. Both pulse pressure variation (PPV) and stroke volume variation (SVV) are reliable indicators for prediction of fluid responsiveness in mechanically ventilated patients. They can be directly measured by Pulse Contour Cardiac Output (PiCCO) technology. The advances in point of care ultrasonography have also played an important role in the determination of intravascular volume status to guide fluid management in the ICU. The size of the inferior vena cava (IVC) and its respirophasic variation can also reflect the intravascular volume status to a certain extent; however, they can also be affected by increased intra-abdominal pressure. Velocity time integral variation (ΔVTI) has a good correlation with the volume responsiveness in critically ill patients (6). It is not affected by intra-abdominal pressure and can be measured at bedside by ultrasonography at any time. Thus, it is a very useful tool in guiding volume management during early fluid resuscitation for SAP.
Dr. Xun Huang (Infection Control Center)
The use of prophylactic antibiotics for SAP is highly controversial. According to the guidelines for the management of AP released by the American College of Gastroenterology and the American Pancreas Association (2,7), prophylactic antibiotics are not recommended for patients with SAP without co-infection. In 2015, the Japanese guidelines for the diagnosis and treatment of pancreatitis proposed that antibiotic prophylaxis within 72 hours may improve the prognosis of SAP and patients with necrotizing pancreatitis. Zhao et al. suggested that, prophylactic use of cephalosporins, quinolones, or carbapenem antibiotics can prevent the translocation of intestinal bacteria in patients with biliary obstruction. For Case 1, antibiotics could be used for a short period of time (within 72 hours) since the patient had biliary SAP. Ceftazidime/sulbactam was used in this patient. The choice of antibiotic was correct, but the medication lasted 7 days, which exceeded the recommended course of treatment. Prolonged use of broad-spectrum antibiotics can increase the risk of superinfection. Case 2 had hyperlipidemic SAP, in whom the use of prophylactic antibiotics should also be based on indicators including presence of sepsis and intra-abdominal pressure. There is a higher risk of intestinal flora translocation in patients with shock in whom the short-term prophylactic use of antibiotics might help.
Dr. Yuhang Ai (Department of Critical Care Medicine)
Many studies have demonstrated that nutritional support therapy plays an important role in improving the clinical outcomes in patients with AP which has become an important component of standardized treatment (8). The EN can protect the functions of the intestinal mucosal barrier through a variety of mechanisms and has been widely used as the first choice for nutritional support. According to the latest guidelines of the American Nutrition Society (9), early EN may be initiated immediately after the 24–48 hours of active fluid resuscitation, and enteral tube feeding with a nasogastric or nasojejunal tube is feasible. Both patients in this article completed active fluid resuscitation in the early stage; however, EN was initiated at day 5 which did not meet the aforementioned nutritional requirement. In particular, serious infection caused by intestinal bacteria occurred 9 days after disease onset, which might be caused by intestinal flora translocation following impaired intestinal barrier function. Of course, the initiation and maintenance of EN is difficult in SAP patients due to increased intra-abdominal pressure and gastrointestinal dysfunction. An objective assessment of the gastrointestinal function helps to guide the effective EN in SAP patients. Unfortunately, there is no highly objective indicator available for assessing the gastrointestinal function in the SAP patients. The expert consensus on acute gastrointestinal injury (AGI) (10) recommended by European Society of Intensive Care Medicine (ESICM) in 2012 is quite helpful.
Dr. Gengwen Huang (Department of Biliopancreatic Surgery)
In the early stage of SAP, the massive release of inflammatory mediators may cause SIRS and MODS. Therefore, the early treatment of SAP should be focused on organ function support in the ICU (11). In the early stage, most SAP patients present with aseptic inflammatory response, and surgical treatment is not required for asymptomatic local complications such as acute peripancreatic fluid collection (APFC) and acute necrotic collection (ANC), so as to avoid puncture-associated complications such as secondary infection and bleeding. For symptomatic patients (e.g., gastrointestinal compression-related symptoms that have affected EN or eating) or in patients with secondary infection, ultrasound to CT guided PCD can be performed. Prokinetic drugs such as intravenous (IV) metoclopramide or IV erythromycin can be used in the early stage to improve gastrointestinal motility or achieve gastrointestinal decompression in patients with increased intra-abdominal pressure. In case of progressively increasing intra-abdominal pressure or nonresponsive abdominal compartment syndrome (ACS), laparotomic decompression can be performed with caution with multidisciplinary approach. Postsurgical incision shall be temporarily covered with a patch or other materials to avoid complications such as intestinal damage (12). Patients with local complications should be closely monitored during the conservative treatment, in particular for the occurrence of co-infection.
Treatment of SAP in late stage
Dr. Yixiong Li (Department of Biliopancreatic Surgery)
The case-fatality rate of AP accompanied with infectious pancreatic necrosis exceeds 30%, and the overall mortality rate could rise up to 80% if sepsis is caused by pancreatic infection. Infectious pancreatic necrosis combined with multiple organ failure has a case-fatality rate of up to 100% if not treated (13). Therefore, the occurrence of infection must be closely monitored during the treatment of SAP patients. The possibility of infection should be considered 7–10 days after disease onset. In SAP patients, the most common site of infection is the necrotic tissue in or around the pancreas, or the fluid collection may be accompanied by infection. Therefore, body temperature, BP, intra-abdominal pressure, WBC count, procalcitonin (PCT), and other signs or laboratory indicators related to infection should be closely monitored during treatment. If an infection is suspected, searching for the etiology with fine needle aspiration is not recommended. Blood culture and CT scan with IV contrast should be performed immediately. An “air bubble sign” on CT scan is suggestive of an infection. Repeated blood cultures can increase the detection rate of pathogens. In Case 1, fever was noted on the 10th day of onset. Although the WBC count was not high, PCT increased rapidly, suggesting the occurrence of bacterial infection. CT revealed an obvious “air bubble sign” in the lesser peritoneal sac (Figure 2), suggesting an infection occurred at this site.
Dr. Xun Huang (Infection Control Center)
For patients with late SAP, if an infection is highly suspected, further examinations are needed to identify the infection and its etiology. Treatment should be based on the culture results of the puncture fluid; however, empirical broad-spectrum antibiotics should be considered while waiting for the culture results. Most common pathogens causing sepsis in these patients are Escherichia coli, bacteroides, enterobacters, Klebsiella pneumoniae, enterococci, and other gram-positive bacteria such as Staphylococcus epidermidis and Staphylococcus aureus. Although there are no adequate evidence concerning the selection of empiric antibiotics for infected pancreatic necrosis and no definite recommendation has been put forth, some studies have explored the permeability of various antibiotics in the pancreatic tissue. Imipenem and ertapenem have good permeability to pancreatic tissue and administration of a single small dose of these drugs can achieve the minimum inhibitory concentration (MIC-90) for the common bacteria (14). For patients with septic shock, the guidelines recommend an empirical combination of antibiotics to cover the most common possible pathogens in a specific region; in the later stages, these antibiotics need to be de-escalated to goal directed therapy based on the results of susceptibility tests as a part of antibiotic stewardship program. Both patients experienced sudden onset high fever and shock in the late stage. Both laboratory tests and imaging examinations indicated the occurrence of infection. Therefore, combination of antibiotics was considered. Since the incidence of carbapenem-resistant Klebsiella pneumoniae infection has risen in our hospital in the recent years, and Case 1 developed septic shock while receiving ceftazidime/sulbactam.
Dr. Weijia Sun (Department of Biliopancreatic Surgery)
For patients with late SAP, if sepsis is clinically detected and CT reveals an “air bubble sign”, a diagnosis of infectious necrosis should be made and surgical treatment should be considered. However, delaying the surgery is sometimes feasible. Once necrosis/infection is confirmed, tailored antibiotic treatment can be initiated immediately. The anti-infective therapy shall be closely monitored, and the surgery can be delayed in the stable patients. Once a diagnosis of infectious pancreatic necrosis (IPN) is confirmed, drainage or debridement should be actively performed in most cases. Uncontrolled infection is associated with almost 100% mortality. In the past, traditional surgical debridement was considered the gold standard for treating IPN. However, in recent years, minimally invasive procedures including percutaneous catheter drainage (PCD), minimal access retroperitoneal pancreatic necrosectomy (MARPN), and retroperitoneal endoscopic debridement for retroperitoneal necrotic tissue have been applied in the clinical settings. Today, an escalation approach represents a mainstream strategy in IPN treatment. Minimally invasive surgeries have the same effectiveness as open surgery and are associated with significant lower incidence of complications. The MARPN-based escalation or other escalation approaches has been adopted by the SAP team in our hospital and have achieved superior results in the treatment of IPN (15). If serious complications such as major bleeding, pancreaticointestinal fistulization, massive residual necrosis or sepsis occur during MARPN, conversion to open surgery should be considered. An open surgery should be considered in case if minimally invasive procedure cannot be performed. In Case 1, sepsis was not effectively treated and shock persisted after PCD. At that time, puncture/drainage alone could not have effectively improve septic shock, and an open surgery should have been considered.
Several issues regarding the management of these two patients were further discussed as follows.
Question 1: How can the volume status and volume responsiveness be accurately determined when the SAP is combined with severely increased intra-abdominal pressure in its early stage?
Expert opinion 1: Dr. Saket Kumar
In patients with Intraabdominal hypertension static parameter of fluid responsiveness is well validated. While dynamic parameters like PPV and SVV are also valid, however, their threshold values will be higher than those without IAH (16). Usual contraindications of low tidal volume, arrhythmia, and spontaneous breathing activity remain the same. Use of PLR in cases of IAH to predict fluid responsiveness in presence of IAH remains controversial (17).
Expert opinion 2: Dr. Marco Vito Marino
Intra-abdominal pressure is a factor which contributes to organ dysfunction. It is also associated with disease severity, organ failure and mortality. However, the monitoring of intra-abdominal pressure is not recommended. The volume responsiveness relies upon traditional markers, such as urine output, blood pressure and pulse oximetry or acid-base balance from an arterial blood gas.
Expert opinion 3: Dr. Hemant Goyal
Increased intraabdominal pressure or intraabdominal hypertension (IAH) is associated with almost 15% of the patients who are diagnosed with SAP. However, the current guidelines American guidelines do not address the utility of surveillance for IAH in SAP. However, several clinical laboratory parameters have been suggested to be associated with higher risk of development of IAH in SAP. Some of them include presence of central obesity, coagulopathy, respiratory failure, edema due to excessive fluid resuscitation/capillary leak, hypotension, and acidosis (pH <7.2). However, none of these parameters have been studied alone or in conjunction with others as an indicator for future development of IAH.
Question 2: If organ failure persists in patients with APFC or ANC, is decompression via puncture feasible?
Expert opinion 1: Dr. Saket Kumar
Drainage of pancreatic abscesses or necrosis is required for the effective control of sepsis. Either percutaneous or endoscopic routes can be utilized to achieve the drainage. With the advent of EUS, the endoscopic route has become more effective as well as safe. Due to thick pus and tenacious necrosis, wide bore drainage is preferred. However, there have been reports of successful management of pancreatic abscess with EUS-guided aspiration and intravenous antibiotic therapy alone, without placement of an indwelling drainage catheter or surgical intervention (18,19).
Expert opinion 2: Dr. Marco Vito Marino
The evaluation of a peri-pancreatic collection depends on the necrotic or sterile nature, and of course on the clinical condition of the patient. The percutaneous retroperitoneal drainage which is feasible in 95% of patients, is the first-step treatment and it is indicated in patients with infected, fluid-predominant collections and when a degree of systemic dysfunction is present. An important alternative is the endoscopic transmural drainage in case of suspected or confirmed (walled-off) infected necrotizing pancreatitis.
Expert opinion 3: Dr. Hemant Goyal
APFCs only require management if they are symptomatic or compress critical structures such as bile duct, bowel or a vessel because the risk of a complication related to drainage are usually high. Initially, the APFC or ANC should be treated conservatively by pain control, enteral nutrition, and antibiotics if there is a clinical suggestion of infection. The most common cause for conservative management failure is refractory infection. This should initially be handled by percutaneous drainage (PCD). However, development of pancreaticocutaneous fistula is a serious complication of PCD, drainage should not be delayed as persistent fever despite antibiotic therapy can result in severe sepsis which can be life-threatening.
APFC are more likely to respond to PCD alone. For ANC in the pancreatic body and tail, the drain should be placed through a retroperitoneally to facilitate subsequent video-assisted retroperitoneal debridement if needed in the future. Smaller (<5 cm) APFC and ANC can be treated via endoscopic retrograde cholangiopancreatography (ERCP) with transpapillary stent placement. Infected ANCs, if do not respond to PCD and antibiotics, then should be surgical debrided by video-assisted retroperitoneal approach or a minimally invasive transgastric route. Open surgical drainage should only be considered as a last resort.
Question 3: Combination therapy is currently recommended for CRE treatment. How long should it last? How can the therapy be de-escalated? Should the combined therapy be stopped directly or de-escalated to mono-therapy?
Expert opinion 1: Dr. Saket Kumar
Combination therapy is an independent predictor of survival in CRE infection. No specific recommendations or guidelines are available. No recommendation of stepping it to monotherapy exists. Local antibiotic stewardship policies and infectious disease specialist should be consulted. More emphasis should be given on patient and staff cohering. Screening and surveillance for carriers and chlorhexidine bathing of patients should be done.
Expert opinion 2: Dr. Marco Vito Marino
The ideal treatment for Carbapenem resistant Enterobacteriace are Tegafycline, fosfomycin and aminoglycoside. No consensus is reached regarding the duration, generally it should be started as a high-dose (in case of Tegafycline is 200 mg, in combination with gentamicin and colistine) and as combination and prolonged strategy (infusion of meropenem over 3–4 hours). Finally reducing according to the response and based on the results of the susceptibility test in order to avoid potential resistant. The new discoveries seem to suggest a de-escalation to mono-therapy before stopping the antibiotics.
Conclusions
SAP has a prolonged disease course which involves multidisciplinary approach during its diagnosis and treatment, while presenting different pathophysiological features in its early and late stages. Treatment of early SAP should be focused on the support and preservation of organ function and should be carried out in the ICU. Serious complications such as infection, hemorrhage, and fistula may occur in the late stage, which should be surgically treated. The involvement of MDT in the treatment of SAP enables the inputs from different departments and ultimately improves the success rate of SAP treatment.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jxym.2019.10.03). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Chinese Society of Pancreatic Surgery. Guidelines on the Diagnosis and Treatment of Acute Pancreatitis (2014 edition). China Journal of Practical Surgery 2015;35:4-7.
- Banks PA, Bollen TL, Dervenis C, et al. Classification of acute pancreatitis-2012: revision of the Atlanta classification and definitions by international consensus. Gut 2013;62:102-11. [Crossref] [PubMed]
- Forsmark CE, Swaroop VS, Wilcox CM. Acute Pancreatitis. N Engl J Med 2016;375:1972-81. [Crossref] [PubMed]
- de-Madaria E, Soler-Sala G, Sánchez-Payá J, et al. Influence of fluid therapy on the prognosis of acute pancreatitis: a prospective cohort study. Am J Gastroenterol 2011;106:1843-50. [Crossref] [PubMed]
- Mounzer R, Langmead CJ, Wu BU, et al. Comparison of existing clinical scoring systems to predict persistent organ failure in patients with acute pancreatitis. Gastroenterology 2012;142:1476-82. [Crossref] [PubMed]
- Muller L, Toumi M, Bousquet PJ, et al. An increase in aortic blood flow after an infusion of 100 ml colloid over 1 minute can predict fluid responsiveness: the mini-fluid challenge study. Anesthesiology 2011;115:541-7. [Crossref] [PubMed]
- Working Group IAP/APA Acute Pancreatitis Guidelines. IAP/APA evidence-based guidelines for the management of acute pancreatitis. Pancreatology 2013;13:e1-e15. [Crossref] [PubMed]
- Li N. Pay attention to nutritional problems in surgical patients. China Journal of Practical Surgery 2012;32:101-3.
- McClave SA, Taylor BE, Martindale RG, et al. Guidelines for the Provision and Assessment of Nutrition Support Therapy in the Adult Critically Ill Patient: Society of Critical Care Medicine (SCCM) and American Society for Parenteral and Enteral Nutrition (A.S.P.E.N.). JPEN J Parenter Enteral Nutr 2016;40:159-211. [Crossref] [PubMed]
- Reintam Blaser A, Malbrain ML, Starkopf J, et al. Gastrointestinal function in intensive care patients: terminology, definitions and management. Recommendations of the ESICM Working Group on Abdominal Problems. Intensive Care Med 2012;38:384-94. [Crossref] [PubMed]
- Kirkpatrick AW, Roberts DJ, De Waele J, et al. Intra-abdominal hypertension and the abdominal compartment syndrome: updated consensus definitions and clinical practice guidelines from the World Society of the Abdominal Compartment Syndrome. Intensive Care Med 2013;39:1190-206. [Crossref] [PubMed]
- Gloor B, Müller C, Worni M, et al. Late mortality in patients with severe acute pancreatitis. Br J Surg 2001;88:975-9. [Crossref] [PubMed]
- Wittau M, Wagner E, Kaever V, et al. Intraabdominal tissue concentration of ertapenem. J Antimicrob Chemother 2006;57:312-6. [Crossref] [PubMed]
- Rhodes A, Evans LE, Alhazzani W, et al. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock: 2016. Intensive Care Med 2017;43:304-77. [Crossref] [PubMed]
- Huang GW, Shen DC. Outcomes of minimal access retroperitoneal pancreatic necrosectomy in the treatment of infectious pancreatic necrosis. China Journal of Practical Surgery 2016;36:1197-9.
- Jacques D, Bendjelid K, Duperret S, et al. Pulse pressure variation and stroke volume variation during increased intra-abdominal pressure: an experimental study. Crit Care 2011;15:R33. [Crossref] [PubMed]
- Renner J, Gruenewald M, Quaden R, et al. Influence of increased intra-abdominal pressure on fluid responsiveness predicted by pulse pressure variation and stroke volume variation in a porcine model. Crit Care Med 2009;37:650-8. [Crossref] [PubMed]
- Jo HG, Amarbat B, Jeong JW, et al. Could Transgastric Endoscopic Ultrasound-Guided Aspiration Alone Be Effective for the Treatment of Pancreatic Abscesses? Clin Endosc 2015;48:345-7. [Crossref] [PubMed]
- Park SW. Is Endoscopic Ultrasound-Guided Drainage Alone Sufficient for the Treatment of Peripancreatic Fluid Collection? Clin Endosc 2017;50:316-7. [Crossref] [PubMed]
Cite this article as: Liu Z, Shen D, Kumar S, Marino MV, Goyal H, Huang G. Clinical report and multidisciplinary team discussion of two fatal cases of severe acute pancreatitis. J Xiangya Med 2019;4:37.