
Association of cytochrome P450 2C19 *2 and *3 variants with type 2 diabetes mellitus in Chinese population
Introduction
According to the latest report from the International Diabetes Federation, 415 million people worldwide suffer from diabetes and this number is projected to rise to 642 million by the year of 2040 (1). Types 2 diabetes is the most common diabetes type characterized by high blood glucose. As a chronic metabolic disorder, it represents the leading cause of macrovascular and microvascular complications including cardiovascular disorders, kidney failure, blindness and lower limb amputation. Although the knowledge in the pathophysiological process of diabetes mellitus has greatly accumulated in recent years, the cellular and molecular mechanisms underlying its genetic pathogenesis remain very limited (2).
Growing evidence are suggesting that a combination of genetic and environmental factors contribute to the development of type 2 diabetes. In recent years, genetic factors including receptors and key enzymes are being frequently researched (3). Of particular interest is the cytochrome P450 gene superfamily involved in drug metabolism as well as synthesis of cholesterol, steroids and other lipids (4). As its major isoform, CYP2C19 acts on 10–15% of drugs in current clinical use, including proton pump inhibitor, platelet aggregation inhibitor, and anti-depressants (5). CYP2C19 also has the capacity of metabolizing polyunsaturated fatty acids (6). Due to its pivotal role in lipid metabolism and proton transfer in mitochondria, CYP2C19 could be a potential candidate for treating type 2 diabetes. CYP2C19 gene consists of 9 exons spanning approximately 90 kb and encodes a protein of 490 amino acids. Approximately 30 variant alleles of CYP2C19 have been identified thus far. Several mutations on CYP2C19 gene have been well documented, such as wild-type allele *1, and mutant alleles *2, *3, *4, *5, *6, *7 and *8. The *1 allele is the wild-type allele that encodes full length of CYP2C19 enzyme. The *2 and *3 alleles are the most common variants and result in a complete loss of enzymatic activity (7,8). However, the relationship of these CYP2C19 variants with type 2 diabetes are not yet well understood. In the present study, we attempted to investigate the association of CYP2C19 polymorphisms with type 2 diabetes in the Chinese population.
Methods
Patients
This study was conducted from 2012 to 2015 at The General Hospital of Western Theater Command. Overall, 575 participants were enrolled in this study, including 226 cases of glycometabolism disorder (GMD) and 349 cases of healthy control with normal glucose metabolism (NGM) (432 males, 143 females; mean age 70±14 years). Among the GMD patients, 62 of them were diagnosed with type 2 diabetes. Subjects with family history of diabetes or coronary heart disease were 10.78% and 58.26%, respectively. Blood samples were collected for further biochemical analysis. Diagnosis of type 2 diabetes mellitus and cardiovascular disordersare made according to WHO diagnostic criteria. The study was approved by the institutional review board of The General Hospital of Western Theater Command and written informed consent was taken from all participants.
Measurements and blood sample collection
The CYP2C19 genotype and biochemical measurements, including total cholesterol (TC), triglyceride (TG) and high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), Cystatin C, fasting blood-glucose (FBG), were performed in accordance with the recommendations proposed by national center for clinical laboratories. Genomic DNA was isolated from whole blood samples using a commercially available DNA isolation kit (TaKaRa, China) according to the manufacturer’s instruction. Then the gene site *1, *2 and *3 were enriched by the PCR method. Finally, the PCR amplification products were hybridized by gene microarrays method. CYP2C19 variants measurements were performed based on single nucleotide polymorphism microarrays (9).
Statistical analysis
Continuous variables were expressed as mean ± standard deviation (SD) and categorical variables were reported as counts and percentages. Analyses of t-tests and chi-square tests were used to examine the differences between control and disease group. Multivariate logistic regression analysis was used to identify independent predictors of type 2 diabetes. Analyses were performed using SPSS version 19.0 statistical software. A value of P<0.05 (two-tailed) was considered significant difference (10). The P value is accurate to three decimal places using SPSS (11).
Result
A total of 575 participants’ CYP2C19 genotypes were performed based on gene microarray (BaiO, Shanghai), and biochemical measurements based on biochemical reaction from Beckman Coulter platform.
Clinical and biochemical characteristics of participants
The clinical profile of the GMD group and the NGM group are summarized in Table 1. As expected, the fast blood glucose, a key criterion of diabetes, was significantly higher in GMD group in comparison with NGM group. Deregulation of lipid metabolism is also a common clinical symptom of type 2 diabetes. Hence, the major lipids, TG was dramatically increased in GMD group, while HDL-C was reduced in GMD group. No significant difference of TC, LDL-C, and Cystatin C were observed between two groups.
Table 1
| Variable | GMD group (n=226) | NGM group (n=349) | t/χ2 value | P |
|---|---|---|---|---|
| Male (%) | 76.1 | 74.5 | 0.008 | 0.928 |
| Age (years) | 71±14 | 68.74±13.27 | 2.309 | 0.021 |
| Coronary heart disease (%) | 61.0 | 56.4 | 0.121 | 0.728 |
| TC (mmol/L) | 4.186±1.241 | 4.139±1.253 | −0.102 | 0.919 |
| TG (mmol/L) | 1.664±1.184 | 1.418±0.725 | 2.596 | 0.010 |
| HDL-C (mmol/L) | 1.110±0.310 | 1.146±0.263 | −2.097 | 0.036 |
| LDL-C (mmol/L) | 2.519±0.986 | 2.409±1.001 | 0.898 | 0.370 |
| Cystatin C (mg/L) | 1.291±0.786 | 1.165±0.502 | 2.410 | 0.016 |
| FBG (mmol/L) | 8.126±3.068 | 5.128±0.502 | 16.71 | 0.000 |
| CYP2C19 *1/*1 (%) | 42.0 | 40.2 | 0.009 | 0.926 |
| CYP2C19 *1/*2 (%) | 38.9 | 38.3 | 0.000 | 0.984 |
| CYP2C19 *1/*3 (%) | 7.1 | 6.8 | 0.002 | 0.961 |
| CYP2C19 *2/*2 (%) | 6.0 | 8.7 | 0.717 | 0.397 |
| CYP2C19 *3/*3 (%) | 0.0 | 0.3 | 0.000 | 1.000 |
| CYP2C19 *2/*3 (%) | 5.0 | 3.8 | 0.085 | 0.771 |
CYP, cytochrome P450; FBG, fasting blood glucose; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; TC, total cholesterol; TG, triglyceride; GMD, glycometabolism disorder; NGM, normal glucose metabolism.
No association of CYP2C19 genotypes with GMD
The levels of TG, HDL-C, Cystatin C, fasting blood-glucose (FBG) were significantly higher in GMD group than in NGM group. However, the association of CYP2C19 genotypes between GMD and NGM group was analyzed and showed no association of any CYP2C19 genotypes (*1/*1, *1/*2, *1/*3, *2/*2, *3/*3, *2/*3) between the two groups. In Table 1, the P value of t-test for all genotypes was over 0.05.
CYP2C19 genotype (*2/*3) is associated with type 2 diabetes
Further, the genotypes of CYP2C19 between the type 2 diabetes group and the GMD without diabetes (GDWD) group were compared. As shown in Table 2, CYP2C19 genotype *2/*3 was found to be significantly higher in the type 2 diabetes group than GDWD group (χ2=4.888, P=0.009). Statistically significant difference of CYP2C19 genotype *2/*3 was also observed between the type 2 diabetes group and the NGM group (χ2=4.888, P=0.000), suggesting that individuals with CYP2C19 *2/*3 mutation may confer a higher risk of developing type 2 diabetes in future.
Table 2
| Variable | Type 2 diabetes (n=62) | GDWD group (n=164) | t/χ2 value | P |
|---|---|---|---|---|
| Male (%) | 67.7 | 78.7 | 0.393 | 0.53 |
| Age (years) | 71±12 | 72±15 | −0.601 | 0.548 |
| Coronary heart disease (%) | 59.60 | 64.02 | 0.000 | 0.997 |
| TC (mmol/L) | 4.442±1.302 | 4.091±1.207 | 1.884 | 0.061 |
| TG (mmol/L) | 1.704±1.149 | 1.649±1.2 | 0.757 | 0.310 |
| HDL-C (mmol/L) | 1.110±0.250 | 1.11±0.33 | −0.73 | 0.942 |
| LDL-C (mmol/L) | 2.754±1.017 | 2.432±0.964 | 2.181 | 0.300 |
| Cystatin C (mg/L) | 1.152±0.377 | 1.343±0.888 | −1.62 | 0.106 |
| FBG (mmol/L) | 7.317±2.629 | 8.417±3.16 | −2.387 | 0.018 |
| CYP2C19 *1/*1 (%) | 35 | 45 | 2.097 | 0.148 |
| CYP2C19 *1/*2 (%) | 33.9 | 40.9 | 1.164 | 0.281 |
| CYP2C19 *1/*3 (%) | 6.5 | 7.3 | 0.000 | 0.984 |
| CYP2C19 *2/*2 (%) | 11 | 4 | 3.534 | 0.060 |
| CYP2C19 *3/*3 (%) | 0 | 0 | 0.000 | 1.000 |
| CYP2C19 *2/*3 (%) | 13 | 2 | 4.888 | 0.009 |
CYP, cytochrome P450; FBG, fasting blood glucose; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; TC, total cholesterol; TG, triglyceride; GMD, glycometabolism disorder; NGM, normal glucose metabolism; GDWD, glycometabolism disorder without diabetes.
Double mutation of CYP2C19 genotypes are highly associated with type 2 diabetes
While the frequencies difference of CYP2C19 wild-type (*1/*1; χ2=2.097, P=0.148) and single mutation genotype (*1/*2 and *1/*3; χ2=1.309, P=0.235) revealed no association between the type 2 diabetes group and the GDWD group. Double mutation (*2/*2, *2/*3 and *3/*3; χ2=12.729, P=0.000) had striking differences between the two groups (Table 3).
Table 3
| Variable | Type 2 diabetes (n=62) | GDWD group (n=164) | χ2 value | P |
|---|---|---|---|---|
| Wild type | ||||
| CYP2C19 *1/*1 (%) | 35 | 45 | 2.097 | 0.148 |
| Single mutation | 1.309 | 0.235 | ||
| CYP2C19 *1/*2 (%) | 33.9 | 40.9 | ||
| CYP2C19 *1/*3 (%) | 6.5 | 7.3 | ||
| Double mutation | 12.729 | 0.000 | ||
| CYP2C19 *2/*2 (%) | 11 | 4 | ||
| CYP2C19 *3/*3 (%) | 0 | 0 | ||
| CYP2C19 *2/*3 (%) | 13 | 2 |
CYP, cytochrome P450; GDWD, glycometabolism disorder without diabetes.
Double mutation of CYP2C19 genotypes are independent risk factor for type 2 diabetes
Remarkably, as shown in Table 4, multivariate logistic regression analysis confirmed CYP2C19 double mutation genotype (*2/*2, *3/*3, *2/*3) as an independent risk factor for type 2 diabetes [odds ratio (OR): 4.960, 95% confidence interval (CI) 1.928–12.760; P=0.000]. Individuals with double mutations appeared to have fivefold increase in their type 2 diabetes risk. Similarly, FBG (OR 1.173; 95% CI, 1.024–1.344; P=0.021) and low-density lipoprotein cholesterol (OR 0.718; 95% CI, 0.531–0.972; P=0.032) were also independent risk factors for type 2 diabetes. Age, coronary heart disease, TC, TG, HDL-C, LDL-C in wild type and single mutation genotype were not associated with the type 2 diabetes.
Table 4
| Independent risk factor | 95% CI | P | OR |
|---|---|---|---|
| Age | 0.986–1.027 | 0.547 | 1.006 |
| Coronary heart disease | 0.663–1.507 | 0.997 | 0.999 |
| TC | 0.623–1.012 | 0.062 | 0.794 |
| TG | 0.755–1.226 | 0.756 | 0.962 |
| HDL-C | 0.391–2.749 | 0.941 | 1.037 |
| LDL-C | 0.531–0.972 | 0.032 | 0.718 |
| Cystatin C | 0.900–2.596 | 0.116 | 1.528 |
| FBG | 1.024–1.344 | 0.021 | 1.173 |
| Wild type (*1/*1) | 0.371–1.162 | 0.148 | 0.656 |
| Single mutation (*1/*2, *1/*3) | 0.410–1.264 | 0.720 | 0.253 |
| Double mutation (*2/*2, *3/*3, *2/*3) | 1.928–12.760 | 0.000 | 4.960 |
FBG, fasting blood glucose; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; TC, total cholesterol; TG, triglyceride; OR, odds ratio; CI, confidence interval.
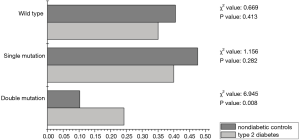
To further validate the association between CYP2C19 double mutation genotype and type 2 diabetes, the association of CYP2C19 double mutation with both cardio cerebrovascular disease and type 2 diabetes were analyzed. As shown in Figure 1, the frequency of CYP2C19 double mutation genotype was dramatically higher in type 2 diabetes (24.2%) than its corresponding nondiabetic control (10.27%) in cardio cerebrovascular disease patients (χ2=6.945, P=0.008). Convincingly, no significant difference was detected in the wild type and single mutation, demonstrating the specificity of double mutation with type 2 diabetes.
Discussion
There are 35 alleles found in the Chinese populations (*2, *3, *8, *11, *13, *14, *16, *19, *23, *27, *29, *31, *33, *34, *36 to *56). *2 and *3 are the main variants among all of CYP2C9. However, the mutation frequency of *2 and *3 is very low (about 2%) in Asian. So CYP2C19 become a focus in this study.
The gene has polymorphisms of some isoforms of CYPs that associated with type 2 diabetes in certain populations. Examples the CYP2C8*3, CYP2C9*2, CYP3A4, CYP2C19*2 and CYP1B1*2 polymorphisms were associated with risk to type 2 diabetes in Indian, Japanese, Mexican and Saudi populations (12,13). Furthermore, the polymorphism CYP2C19*2 has been described to be associated with susceptibility to metabolic syndrome in south Portuguese population.
CYP2C19 is located within a cluster of cytochromes P450 genes on chromosome 10q24, which contains nine exons and eight introns (14). The gene encodes a 490-aa long protein of approximately 56 kDa, which is a member of the cytochrome P450 superfamily of enzymes. CYP2C19 is a clinically significant drug-metabolizing enzyme and its genotyping and phenotyping information have the potential to improve drug safety and efficacy (15). At least 27 variant alleles for CYP2C19 have been identified, with the most extensively described being CYP2C19*2, CYP2C19*3 in Chinese population (16).
CYP2C19*2 has been shown to be a G681A transition exon 5 of wild-type CYP2C19*1. This variant results in a wrong reading frame and produces a truncated protein. The CYP2C19*3, on the other hand, involves a G636A variant in exon 4, that encode a premature stop codon and a truncated protein (17). In clinical application, six CYP2C19 genotypes (CYP2C19*1/*1, *1/*2, *1/*3, *2/*2, *2/*3 and *3/*3,) have been observed in 99% of Chinese population. In the present study, double mutations of CYP2C19 (*2/*2, *2/*3 and *3/*3) are highly associated with type 2 diabetes, suggesting that the normal function of CYP2C19 may be required for proper glucose metabolism.
According to earlier studies, the frequency of CYP2C19 genotypes was variable among populations. For example, the frequencies of six CYP2C9 genotypes were 43.5%, 42.9%, 0.3%, 12.7%, 0.6% and 0.0% in South Asia, while the genotype frequencies were 44.9%, 41.1%, 4.7%, 7.0%, 1.8% and 0.6% in Southeast Asia (18). In this study, the genotype frequencies of CYP2C19 in Chinese population were 41.6%, 38.9%, 7.0%, 7.8%, 4.5% and 0.2%, respectively. Consistent with their findings, our results reiterated a great difference in the genotype frequencies of CYP2C19 from different regions of Asia.
Previously, it has been documented that CYP2C19 is associated with the occurrence of coronary heart disease and stroke. A positive association between diabetes mellitus and vascular dementia was also established (19). Here our work further demonstrated that CYP2C19 double mutation genotype is specifically related to type 2 diabetes, but not cardio cerebrovascular disease. This was confirmed by a comparative analysis of double mutant genotypes in cardio cerebrovascular disease patients with the type 2 diabetes patients showing a significant higher frequency of double mutant genotypes. Multivariate logistic regression analysis also indicated that the CYP2C19 double mutation genotype is an independent risk factor for type 2 diabetes, and individuals are 5-fold more likely to develop type 2 diabetes. We therefore propose that CYP2C19 double mutation is a specific genotype related to increased type 2 diabetes susceptibility in Chinese.
Indeed, type 2 diabetes mellitus results from the interaction between genetic and environmental factors, leading to heterogeneous and progressive pancreatic β-cell dysfunction (20). In particular, glucocorticoids are known to exert deleterious effects on the glucose metabolism, leading to a wide range of alterations from insulin resistance to diabetes. Under stress condition, glucocorticoids also regulate cytochrome P450 family (21). Since CYP2C19 expression is regulated by constitutive and rostane receptor (CAR) involved in glucocorticoids synthesis, it is reasonable to propose that there are certain interactions between CYP2C19 double mutant genotypes and type 2 diabetes through some of these receptors in charge of cholesterol regulation and bile acid.
Conclusions
Taken together, the association between CYP2C19 double mutation genotype and type 2 diabetes was strikingly significant; suggesting that double mutation genotype directly translates into higher type 2 diabetes susceptibility. As an independent risk factor for type 2 diabetes, individuals with double mutation genotype are also five-fold more susceptible to develop type 2 diabetes, as compared with those with the wild-type or single mutation genotype. Furthermore, GMD patients with CYP2C19 double mutation genotype are most likely to deteriorate into type 2 diabetes. Hence, the potential role of CYP2C19 polymorphism in the development of type 2 diabetes underscores the importance of genomics towards preventing this growing epidemic.
Acknowledgments
Authors are grateful to the staff of the Department of Medical Laboratory (The General Hospital of Western Theater Command) for their technically assistance throughout this study. We also thank all the volunteers who have participated in this research.
Funding: This study was supported by applied basic research project of the Science and Technology Department in Sichuan (grant No. 2013JY0173) and The General Hospital of Western Theater Command Routine Management Project of Science (grant No. 2016KC04).
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jxym.2019.08.01). The authors have no conflicts of interest to declare.
Ethical Statement:
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Jaacks LM, Siegel KR, Gujral UP, et al. Type 2 diabetes: A 21st century epidemic. Best Pract Res Clin Endocrinol Metab 2016;30:331-43. [Crossref] [PubMed]
- Tanaka K, Tsuji I, Tamakoshi A, et al. Diabetes mellitus and liver cancer risk: an evaluation based on a systematic review of epidemiologic evidence among the Japanese population. Jpn J Clin Oncol 2014;44:986-99. [Crossref] [PubMed]
- Uma Jyothi K, Reddy BM. Gene-gene and gene-environment interactions in the etiology of type 2 diabetes mellitus in the population of Hyderabad, India. Meta Gene 2015;5:9-20. [Crossref] [PubMed]
- Guengerich FP, Waterman MR, Egli M. Recent Structural Insights into Cytochrome P450 Function. Trends Pharmacol Sci 2016;37:625-40. [Crossref] [PubMed]
- Lewis DF. Human cytochromes P450 associated with the phase 1 metabolism of drugs and other xenobiotics: a compilation of substrates and inhibitors of the CYP1, CYP2 and CYP3 families. Curr Med Chem 2003;10:1955-72. [Crossref] [PubMed]
- Westphal C, Konkel A, Schunck WH. Cytochrome p450 enzymes in the bioactivation of polyunsaturated Fatty acids and their role in cardiovascular disease. Adv Exp Med Biol 2015;851:151-87. [Crossref] [PubMed]
- Oestreich JH, Best LG, Dobesh PP. Prevalence of CYP2C19 variant alleles and pharmacodynamic variability of aspirin and clopidogrel in Native Americans. Am Heart J 2014;167:413-8. [Crossref] [PubMed]
- Desta Z, Zhao X, Shin JG, et al. Clinical significance of the cytochrome P450 2C19 genetic polymorphism. Clin Pharmacokinet 2002;41:913-58. [Crossref] [PubMed]
- Dodgen TM, Hochfeld WE, Fickl H, et al. Introduction of the AmpliChip CYP450 Test to a South African cohort: a platform comparative prospective cohort study. BMC Med Genet 2013;14:20. [Crossref] [PubMed]
- Chang K, Qiu F, Chen M, et al. Engineering the MEP pathway enhanced ajmalicine biosynthesis. Biotechnol Appl Biochem 2014;61:249-55. [PubMed]
- Ariyoshi K, Okuya S, Kunitsugu I, et al. Ultrasound analysis of gray-scale median value of carotid plaques is a useful reference index for cerebro-cardiovascular events in patients with type 2 diabetes. J Diabetes Investig 2015;6:91-7. [Crossref] [PubMed]
- Yamada Y, Matsuo H, Watanabe S, et al. Association of a polymorphism of CYP3A4 with type 2 diabetes mellitus. Int J Mol Med 2007;20:703-7. [PubMed]
- Elfaki I, Mir R, Almutairi FM, et al. Cytochrome P450: Polymorphisms and Roles in Cancer, Diabetes and Atherosclerosis. Asian Pac J Cancer Prev 2018;19:2057-70. [PubMed]
- Lamoureux F, Duflot T, Woillard JB, et al. Impact of CYP2C19 genetic polymorphisms on voriconazole dosing and exposure in adult patients with invasive fungal infections. Int J Antimicrob Agents 2016;47:124-31. [Crossref] [PubMed]
- Tabata N, Hokimoto S, Akasaka T, et al. Patients with both CYP2C19 loss-of-function allele and peripheral endothelial dysfunction are significantly correlated with adverse cardiovascular events following coronary stent implantation. J Cardiol 2016;67:104-9. [Crossref] [PubMed]
- Hu LM, Dai DP, Hu GX, et al. Genetic polymorphisms and novel allelic variants of CYP2C19 in the Chinese Han population. Pharmacogenomics 2012;13:1571-81. [Crossref] [PubMed]
- Tatarunas V, Jankauskiene L, Kupstyte N, et al. The role of clinical parameters and of CYP2C19 G681 and CYP4F2 G1347A polymorphisms on platelet reactivity during dual antiplatelet therapy. Blood Coagul Fibrinolysis 2014;25:369-74. [Crossref] [PubMed]
- Gaikwad T, Ghosh K, Shetty S. VKORC1 and CYP2C9 genotype distribution in Asian countries. Thromb Res 2014;134:537-44. [Crossref] [PubMed]
- Ahtiluoto S, Polvikoski T, Peltonen M, et al. Diabetes, Alzheimer disease, and vascular dementia: a population-based neuropathologic study. Neurology 2010;75:1195-202. [Crossref] [PubMed]
- Di Dalmazi G, Pagotto U, Pasquali R, et al. Glucocorticoids and type 2 diabetes: from physiology to pathology. J Nutr Metab 2012;2012:525093. [Crossref] [PubMed]
- Hukkanen J, Vaisanen T, Lassila A, et al. Regulation of CYP3A5 by glucocorticoids and cigarette smoke in human lung-derived cells. J Pharmacol Exp Ther 2003;304:745-52. [Crossref] [PubMed]
Cite this article as: Chang K, Chen Y, Xiong J, Ren J, Jiang Z. Association of cytochrome P450 2C19 *2 and *3 variants with type 2 diabetes mellitus in Chinese population. J Xiangya Med 2019;4:34.


