
Systemic AL amyloidosis with high bone marrow plasma cells (BMPCs) infiltration: a case report and literature review
Introduction
Light chain (AL) amyloidosis is a complex and often fatal disease caused by misfolded protein deposition characterized by monoclonal immunoglobulin light-chain fibrils in tissues and organs (1). This light chain protein, usually produced by a small and indolent plasma cell clone, is responsible for positive staining with Congo red when observed under polarized light, a gold standard for the diagnosis of AL amyloidosis (2). According to the extent of the amyloid deposits involved, AL amyloidosis classified into the localized AL amyloidosis which only involving single tissue or organ, and the systemic AL amyloidosis which may affect all organs of the body except for central nervous system and generate a wide range of clinical symptoms (3). System amyloidosis is the most common type in western countries, with an estimation of about 9 cases per million inhabitants per year (4). There is a consensus that AL amyloidosis patients with hypercalcemia, renal failure, anemia, and lytic bone lesions due to clonal expansion of plasma cells (CRAB criteria) show the high possibility of concurrent MM. The median percentage of bone marrow plasma cells (BMPCs) among patients with AL amyloidosis is 7% to 10% (5,6). Kourelis et al. discovered that AL amyloidosis patients with more than 10% BMPCs had a prognosis similar to that of AL amyloidosis patients with CRAB, and should, therefore, be considered as AL amyloidosis with MM (7). Another study found that the shorter overall survival (OS) of patients with higher BMPCs was probably related to a higher cardiac involvement instead of relating to the clonal plasma cell expansion (8). As a result, the proportion of BMPCs was not a criterion to distinguish AL amyloidosis from multiple myeloma (MM) but indicated a poor prognosis. Here we reported a case of systemic AL amyloidosis with high BMPCs infiltration, cardiac failure and poor prognosis, the involved organs including skin, tongue, kidney, heart, autonomic nervous system and the small vessels of the body.
Case representation
A 48-year-old female patient, previously healthy, began with edema of lower extremities without precipitating factors since early 2017. The patient did not pay attention to it at first. In April 2017, periorbital ecchymosis appeared (Figure 1A). She went to a local hospital and found her urinary protein was (2+), and related examination showed hypoalbuminemia, massive proteinuria, and hyperlipemia. She was diagnosed with nephrotic syndrome and did not know whether it was primary or secondary. She received glucocorticoid, diuretics, albumin supplementation, and other supportive treatment, but her symptoms progressed. In August 2017, her tongue was gradually enlarged (Figure 1B), accompanied by dysphagia. About ten days before admitted to our hospital, her edema became aggravated and developed to the whole body, along with shortness of breath, dizziness, and amaurosis on exertion. She was admitted to our hospital on January 3, 2018.
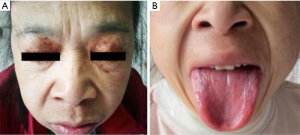
On physical examination, her blood pressure was 88/50 mmHg from both arms. Heart rate, respiratory rate, and temperature were within normal range. She had periorbital ecchymosis, noticeable big and red tongue with little tongue fur, and pitting edema of both lower limbs.
She underwent a systemic medical examination showed hypoalbuminemia and hyperlipemia. The quantification of proteinuria was 7.26 g/24 hour mainly composed of albumin. Immunofixation electrophoresis of both serum and urine revealed a positive λ-type light chain. NT-proBNP elevated to 6,271 pg/mL. Electrocardiogram (ECG) indicated the low voltage in limb leads, poor R wave progression from V1 to V6, clockwise rotation and right axis deviation. Echocardiography showed inhomogeneous ventricular thickening, with normal ejection fraction (58%). The 24-hour ambulatory blood pressure reported persistent hypotension. Renal and skin biopsy (Figure 2A,B,C) presented a positive Congo red staining. Electron microscopic examination of renal biopsy confirmed the deposition of amyloid substance (Figure 2D) and Immunofluorescence staining of the renal revealed positive λ-type light chain (Figure 2E). Bone marrow examination and flow cytometry showed high plasma cells ratio (17%) (Figure 2F) with aberrant immunophenotyping (Figure 2G). The hemoglobin, electrolyte, creatinine, and transaminase were within the normal range. The karyotypic analysis and fluorescence in situ hybridization of the bone marrow excluded the abnormality in cytogenetics and molecular biology. The chest X-ray was normal, and bone SPECT did not reveal lytic lesions (Figure 2H).
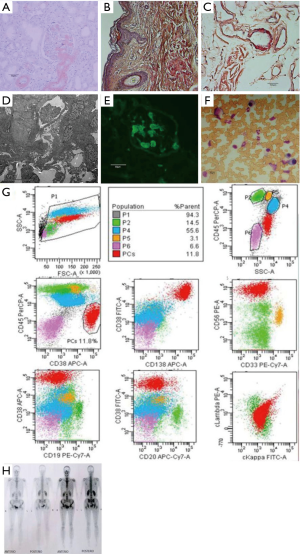
We diagnosed this patient as systemic AL amyloidosis with high BMPCs infiltration. On January 13, 2018, she was given the melphalan-Prednisone regimen for therapy considering her economic condition. Unfortunately, she passed away because of cardiac failure on March 24, 2018.
iMDT discussion
Discussion among physicians from the Department of Hematology, Department of Nephrology and Department of Oncology at Xiangya Hospital
Systemic AL amyloidosis is a rare disease which can involve multiple organs and tissues, especially kidney, heart, liver, nerve, gastrointestinal tract, lung, and soft tissues. We reported a case of systemic AL amyloidosis with high BMPCs infiltration (17%), which affected multiple tissues and organs, and led to a series of clinical symptoms. Differentiating it from MM is necessary. Certain clinical manifestations and examinations are significant. Positive staining with Congo red is required for the diagnosis of amyloidosis. MM usually manifests CRAB symptoms, and even the smoldering myeloma has its typical gene alterations such as t(4,14), del17p13. Proteinuria in AL amyloidosis mainly composed of albumin, but in MM manifests as light-chain protein. Furthermore, lambda clones dominate kappa ones by 4:1 in AL amyloidosis, unlike the 2:3 ratio in myeloma (9). As reported that higher BMPCs infiltration in patients with AL amyloidosis was associated with multiple organs damages and poor prognosis. Early diagnosis and appropriate treatment should be adopted timely. These patients should be treated as the patients of AL amyloidosis coexisting with MM. Eradicating the production of the AL amyloid fibril precursor protein as quickly as possible, minimizing treatment-related toxicity and preserving organ function are the primary goals of treatment (10). Supportive treatment is also fundamental especially for the patients with cardiac involvement due to the short lifetime. Patients with AL amyloidosis can be sorted into different categories according to the 2004 and 2012 Mayo AL amyloidosis staging system. Autologous stem cell transplantation and combinational chemotherapy regimens are the main treatments modalities. For more than 40 years, alkylating agents, and more specifically, melphalan and corticosteroids were the mainstay treatment regimen for AL amyloidosis (11). Immunomodulatory agents (such as thalidomide, Lenalidomide, and pomalidomide), proteasome inhibitor-based regimens were also applied in clinical practice according to the condition of the patients (12). The survival of AL amyloidosis depends on the extent and severity of organs involvement at their initial diagnosis and treatment (3). We failed to stratify the risk factors for this patient according to the Mayo AL amyloidosis staging system due to lacking some necessary examinations [such as the difference between the involved and uninvolved free light chain (dFLC) and cardiac troponin T]. However, both of the high BMPCs infiltration and heart involvement indicated a poor prognosis. Eventually, she passed away 70 days after treatment because of cardiac failure even though she adopted melphalan-Prednisone therapy.
Some questions were further discussed as follows
The patient was diagnosed as systemic AL amyloidosis or MM, is the diagnosis correct?
Expert opinion 1: Dr. Morie A. Gertz
This patient has classic AL amyloidosis. There were no crab criteria to suggest active or overt MM. The median number of plasma cells at diagnosis in AL amyloidosis is 10 this is only slightly above the media. I would not consider this patient to have MM.
Expert opinion 2: Dr. Hiroki Kobayashi
The diagnosis of MM requires 10% or more clonal plasma cells on bone marrow examination or a biopsy-proven plasmacytoma plus the presence of one or more myeloma-defining events (13). According to the manuscript, the patient did not have hypercalcemia, anemia, cast nephropathy, and bone disease (myeloma-defining events). Therefore, I think that we could not diagnose her as MM. I think that the patient should be diagnosed as systemic AL amyloidosis with concurrent smouldering MM.
What are the key points for differentiating MM from AL amyloidosis in patients with high BMPC infiltration?
Expert opinion 1: Dr. Morie A. Gertz
This patient had nephrotic syndrome, eriorbital purpura, tongue enlargement, and cardiomyopathy. All of these features conform to light chain amyloidosis rather than MM. The diagnosis of MM cannot simply be made based on the percentage of plasma cells in the bone marrow.
Expert opinion 2: Dr. Hiroki Kobayashi
AL amyloisis can coexist with MM (13). I think that we should not distinguish MM from AL amyloidosis. But we can distinguish AL amyloidosis with concurrent MM from AL amyloidosis alone, according to the presence of myeloma-defining events.
Is the bone SPECT significant to differentiate MM from AL amyloidosis?
Expert opinion 1: Dr. Morie A. Gertz
Bone imaging is important since lytic lesion should not be seen in amyloidosis. In this instance, it was useful to reaffirm the diagnosis of amyloidosis.
Expert opinion 2: Dr. Hiroki Kobayashi
The bone SPECT can detect myeloma-related bone disease. Therefore, the bone SPECT can contribute to diagnosis of the presence of MM. As I mention previously, we can distinguish AL amyloidosis with concurrent MM from AL amyloidosis without MM, when we used the bone SPECT. PET-CT and MRI can also detect the presence of myeloma-related bone disease.
How to sensitively identify and diagnose AL patients begin with nephrotic syndrome?
Expert opinion 1: Dr. Morie A. Gertz
The workflow of a nephrologist seeing a patient with proteinuria should include measurement of kappa and lambda free light chains in the serum. The presence of a light chain abnormality with heavy proteinuria should raise either amyloidosis or light chain deposition disease.
Expert opinion 2: Dr. Hiroki Kobayashi
It is difficult to diagnose renal AL amyloidosis early. I think what is important to diagnose renal AL amyloidosis is free-light chain assay (14). In our hospital, nephrologists routinely examine serum free light chain of the patients with nephrotic syndrome. If the patient had abnormal free-light chain ratio, they would consult us, and the skin and bone marrow biopsy would be considered. If the Congo red stain of skin and bone marrow was negative, renal biopsy would consider.
Conclusions
Due to the very rare incidence of AL amyloidosis with high BMPCs infiltration and their unique clinical manifestation and prognosis, we suggested that AL amyloidosis with more than 10% clonal plasma cells in bone marrow should be distinguished from simple AL amyloidosis, AL amyloidosis combines with MM, and MM secondary amyloidosis. In this case, we diagnosed the patient as systemic AL amyloidosis with high BMPCs infiltration which had a poor prognosis. Although both AL amyloidosis and MM are plasma cell malignancy, the correct diagnosis can clear the disease type and guide treatment.
Acknowledgments
Funding: This work was supported by National Natural Science Foundation of China (Grant No. 81770184). We also thank Dr. Moshe E. Gatt for critical reading of the manuscript and giving important advices.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jxym.2019.07.01). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this Case report and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Comenzo RL. Systemic immunoglobulin light-chain amyloidosis. Clin Lymphoma Myeloma 2006;7:182-5. [Crossref] [PubMed]
- Picken MM. Amyloidosis-where are we now and where are we heading? Arch Pathol Lab Med 2010;134:545-51. [PubMed]
- Khan MF, Falk RH. Amyloidosis. Postgrad Med J 2001;77:686-93. [Crossref] [PubMed]
- Kyle RA, Linos A, Beard CM, et al. Incidence and natural history of primary systemic amyloidosis in Olmsted County, Minnesota, 1950 through 1989. Blood 1992;79:1817-22. [PubMed]
- Kyle RA, Gertz MA. Primary systemic amyloidosis: clinical and laboratory features in 474 cases. Semin Hematol 1995;32:45-59. [PubMed]
- Kumar S, Dispenzieri A, Lacy MQ, et al. Revised prognostic staging system for light chain amyloidosis incorporating cardiac biomarkers and serum free light chain measurements. J Clin Oncol 2012;30:989-95. [Crossref] [PubMed]
- Kourelis TV, Kumar SK, Gertz MA, et al. Coexistent multiple myeloma or increased bone marrow plasma cells define equally high-risk populations in patients with immunoglobulin light chain amyloidosis. J Clin Oncol 2013;31:4319-24. [Crossref] [PubMed]
- Palladini G, Dispenzieri A, Gertz MA, et al. New criteria for response to treatment in immunoglobulin light chain amyloidosis based on free light chain measurement and cardiac biomarkers: impact on survival outcomes. J Clin Oncol 2012;30:4541-9. [Crossref] [PubMed]
- Dispenzieri A, Merlini G. Immunoglobulin Light Chain Systemic Amyloidosis. Springer International Publishing, 2016.
- Comenzo RL, Reece D, Palladini G, et al. Consensus guidelines for the conduct and reporting of clinical trials in systemic light-chain amyloidosis. Leukemia 2012;26:2317-25. [Crossref] [PubMed]
- Kyle RA, Gertz MA, Greipp PR, et al. A trial of three regimens for primary amyloidosis: colchicine alone, melphalan and prednisone, and melphalan, prednisone, and colchicine. N Engl J Med 1997;336:1202-7. [Crossref] [PubMed]
- Zumbo G, Sadeghi-Alavijeh O, Hawkins PN, et al. New and developing therapies for AL amyloidosis. Expert Opin Pharmacother 2017;18:139-49. [Crossref] [PubMed]
- Rajkumar SV, Dimopoulos MA, Palumbo A, et al. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol 2014;15:e538-48. [PubMed]
- Gillmore JD, Wechalekar A, Bird J, et al. Guidelines on the diagnosis and investigation of AL amyloidosis. Br J Haematol 2015;168:207. [Crossref] [PubMed]
Cite this article as: Wang HW, Tang R, Xiao XC, Liu W, Gertz MA, Kobayashi H, Zeng H. Systemic AL amyloidosis with high bone marrow plasma cells (BMPCs) infiltration: a case report and literature review. J Xiangya Med 2019;4:30.


