
Assessment of risk factors for cardiovascular diseases among patients attending cardiac clinic at a referral hospital in Tanzania
Introduction
Mortality and morbidity rates due to cardiovascular diseases (CVDs) are escalating worldwide, with disproportionately significant worse outcomes in the developing countries, due to rapid health and nutrition transitions caused by urbanization and globalization. More than 17.92 million people died in 2015 due to CVDs, with the highest death rate of 9.4 million recorded in men than 8.5 million in women (1,2). Coronary heart disease (CHD) ranks as the highest cause of deaths among all CVDs, accounting for more than 8.9 million deaths worldwide (3). The prevalence of CHD is increasingly uneven in different regions of the world, due to inadequate and lack of better health services (4). CHD is considered as a single cause of mortality and morbidity across all gender groups in developed and developing countries (4). Moreover, CHD was previously not given enough intervention priority in many countries from the Sub-Saharan region, due to lack of strong epidemiological studies that would determine true burden of disease (5,6). Findings from the recent [2008–2013] studies predicted that the growing burden of CHD in the region will exceed that observed in other world regions if no appropriate preventive measures will be taken to alleviate the problem (7). Similarly, hypertension (HTN) and HTN-related diseases are increasing the number of inpatients and outpatients in referral hospitals in Tanzania, posing healthcare challenges in primary and secondary healthcare, due to inadequate resources for their treatment and management (8,9).
The growing CHD mortality rate is exacerbated by the increased levels of blood pressure and other CVD risk factors. HTN is one of the leading risks for global mortality, accounting for 13% of the total deaths (10), and is attributing to more than 45% of all CHD deaths and 51% deaths due to stoke worldwide (11). Untreated HTN results in more health complications, such as myocardial infarction (MI), renal diseases, stroke, heart failure and premature deaths (11). Treatment of blood pressure has been associated with more than 40% and 16% reduction of stroke and MI risk factors, respectively (12). The ultimate goal of managing HTN is to achieve target control and prevent the development of other HTN-related complications (13).
The management of HTN and HTN-related diseases, including CHD, usually varies from one country to another, as it depends on the capacity of the country to utilize the available resource for prevention and control of the diseases (14). For example, lowering of blood pressure together with the reduction of other CVD risk factors has been associated with 50% reduction of CVD mortality in some of the high-income countries (15). As a rule of thumb, the World Health Organization and the International Society of Hypertension recommend management of HTN by monitoring other CVD risk factors, patient-centered lifestyle modification, damage organ and their related clinical signs (14,16), rather than relying on taking blood pressure measurement alone. Factors that need to be monitored during management includes levels of systolic and diastolic blood pressure, gender, age, smoking, blood cholesterol, history of CVD, obesity, physical activity, and diabetes.
Lifestyle modification is one of the components of HTN management. Modifiable lifestyle risk factors, despite effectively delaying or preventing the onset of HTN, they also contribute to the reduction of blood pressure in patients with HTN and sometimes they may reduce the need for antihypertensive therapy (17,18). There is strong evidence of regular physical activity and protection against HTN (18). Regular physical activity is associated with a reduction of 3.2 and 2.7 mmHg systolic and diastolic blood pressure, respectively. However, for patients with poorly controlled HTN (systolic ≥180 mmHg and diastolic ≥100 mmHg), physical exercise should be suspended until their blood pressure stabilized (17,18). It is also recommended to engage in any physical exercise rather than none. On the other hand, weight reduction is associated with a reduction in blood pressure, blood sugar, and reduced CVD risks (19). Evidence from meta-analysis showed that reduction of 5.1 kg of body weight by means of energy restriction and increased physical activity reduces systolic and diastolic blood pressure by 4.4 and 3.6 mmHg, respectively (20). Diet modification that emphasizes on adequate intake of fruits and vegetables, whole grains, low dietary fats, low intake of dietary sodium and salt reduction has shown a positive impact in the reduction of blood pressure in normotensive and hypertensive patients (17,21).
Furthermore, inflammatory biomarkers for CVD such as lipids, high-sensitive C-reactive protein (CRP) and troponins play an important role in the diagnosis, monitoring of diseases progression, and decision making regarding the management of cardiovascular events (22,23). For example, CRP has been used to assess CVD risks, heart attack, stroke (23) and it has been identified as a single predictor of the risk of CVD events (24).
Despite the growing trends of CVD cases in a hospital setting in Tanzania, their management is only based on measurements of a few intermediate risk factors. Up to date, no study had been conducted in Tanzanian hospital settings to evaluate CVD risk factors among patients attending cardiac clinics, to see how they respond to current treatment. Therefore, this study was carried out to quantify lifestyle risk factors and biomarkers associated with HTN and CHD among patients attending cardiac clinics at Kilimanjaro Christian Medical Centre (KCMC) referral hospital in Kilimanjaro region-Tanzania.
Methods
Study settings
This study was conducted at KCMC located in Kilimanjaro region-Tanzania. KCMC is a referral hospital which serves for over 15 million people in the northern, eastern and central zone of Tanzania. The hospital has a capacity to serve for 500–800 inpatients per day.
Study design and sampling method
This was a cross-sectional hospital-based study to determine the prevalence of lifestyle risk factors among CVD patients attending the cardiac clinic at KCMC referral hospital. The study was conducted between April and July 2018. The purposeful sampling method was employed to select all patients who met the study selection criteria.
Inclusion and exclusion criteria
Inclusion criteria
Adults aged ≥35 years diagnosed with CHD and HTN who attended the Cardiac clinic at KCMC referral hospital from April to July 2018. The study participants voluntarily consented to participate in the study.
Exclusion criteria were
Children (including those with congenital heart diseases), pregnant women and patients with CHD and HTN aged ≥35 years who did not consent to take part in the study.
Assessment of socio-demographic characteristics and lifestyle risk factors
A structured questionnaire with closed questions was adopted from the WHO STEPwise survey (25) and translated to Kiswahili language (national language). The questionnaire was then administered to all participants. The following information was collected: socio-demographic information, lifestyle risk factors and family history of HTN and CHD. The assessed socio-demographic characteristics were: age, gender, marital status, and education level and occupation status. Education level was categorized as primary level, secondary level, higher education learning and uneducated. Marital status (married and no partners), occupation (formal employment, self-employed and unemployed). Lifestyle risk factors included current/history of smoking for the past 5 years (categorized as smoker or non-smoker), history of alcohol use (categorized as current alcohol user or non-alcoholic user), physical activity (categorized as physical exercise at least 2 days per week minimum for 30 minutes or no physical activity), and family history of either HTN or CHD (defined as having at least close relative (father, mother, sister or brother) diagnosed with ether HTN/CHD or both HTN and CHD).
Anthropometric measurements
Weight in kilogram was taken in light clothing by using calibrated weighing scale machine (Seca, Germany), with 150 kg capacity and the accuracy of 0.5 kg. The patient was requested to remain with minimal clothes, remove shoes and excess weight in the pockets before measurements were taken. Height was measured in centimeter (cm) by calibrated stadiometer (Leicester stadiometer) of 0.1 cm accuracy, with the subject standing against the vertical wall, heels together, shoulders and head touching the wall surface and after removal of shoes. Body mass index (BMI) was then calculated by the following formula [BMI = weight (kg)/height (m2)]. BMI was categorized as underweight (<18.5), normal (18.5–24.9), overweight (25.0–29.9) and obese (≥30.0) (26).
Blood pressure measurements
Blood pressure measurement was conducted by the trained clinical officer upon arrival of the patient and after resting for 10–15 minutes. Automatic digital sphygmomanometer with automatic inflation (Life Brand™ BM60) was used to measure blood pressure while the patient seated and relaxed with the left hand at the level of the heart. Three systolic and diastolic blood pressure readings were taken on the left upper arm of the patient. Average systolic and diastolic blood pressure was used in the analysis. Systolic and diastolic blood pressure measurements were used to classify HTN in accordance with the Seventh Joint National Committee (27) (Table 1).
Table 1
| Classification | Systolic BP (mmHg) | Diastolic BP (mmHg) |
|---|---|---|
| Normal | <120 | <80 |
| Pre-hypertension | 120–139 | 80–89 |
| Hypertension stage I | 140–159 | 90–99 |
| Hypertension stage II | ≥160 | ≥100 |
Blood sample collection
Blood samples for plasma glucose, serum ALT, CRP, high-density lipoprotein cholesterol (HDL-C), and low-density lipoprotein cholesterol (LDL-C) concentration measurements were obtained by a trained clinician. For each patient, 10 mL of venous blood samples were drawn from the arm and transferred to EDTA (ethylenediaminetetraacetic acid) tube. Blood samples were then taken to a clinical research laboratory at KCMC referral hospital for further analysis procedures. Blood samples were centrifuged at the 3,000-rpm machine (Roche Germany) for 5 minutes at 4 °C. Clarified serum and plasma samples were then pipetted and poured into Eppendorf storage tubes (5 mL), followed by freezing at −20 °C.
Analysis of biomarkers
Before analysis, plasma and serum blood samples were mixed thoroughly by using vortex mixer. Form each sample, 10 µL was pipetted and poured into Microvatte tubes. Plasma blood glucose, HLD-C, LDL-C, and ALT samples were loaded into Cobas Integra 400 plus analyzer (Roche Diagnostics, Germany). Serum blood for measuring CRP concentration was loaded into fully-auto chemiluminescence immunoassay (CLIA) analyzer (MAGLUMI 800), Shenzhen New Industries Biomedical Engineering Co., Ltd. (Snibe Diagnostic, China). According to laboratory protocols, values (concentrations) of studied biochemistry markers were categorized as indicated in Table 2.
Table 2
| Biomarkers | Descriptor |
|---|---|
| Plasma glucose (mmol/L) | |
| 3.5–6.5 | Normal |
| >6.5 | Hyperglycemia/diabetes |
| HDL-C (mmol/L) | |
| >1.15 | Normal-men |
| >1.68 | Normal-female |
| 0.90–1.45 | Moderate risk-men |
| 1.15–1.68 | Moderate risk-female |
| <0.90 | High risk-men |
| <1.15 | High risk-female |
| LDL-C (mmol/L) | |
| <2.59–3.34 | Normal |
| >3.34 to ≥4.92 | High risk |
| ALT (IU/L) | |
| <31 | Normal-male |
| <19 | Normal-female |
| >31 | High risk-male |
| >19 | High risk-female |
| CRP (mmol/L) | |
| <1 | Normal |
| 1–3 | Moderate risk |
| >3 | High risk |
HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; ALT, alanine aminotransferase, CRP, C-reactive protein.
Statistical analysis
Data were entered into Microsoft Excel 2013, then sorted, coded, and cleaned. The analysis was done using SPSS version 20.0 (IBM). Descriptive statistics were used to analyze the frequency and percentages of socio-demographics, lifestyle characteristics, and biomarkers for HTN and CHD. Pearson’s chi-square (χ2) test was used to determine the association between risk factors with HTN and CHDs. Independent variables included in the analysis were: gender, age, education level, occupation, marital status, BMI, blood pressure, physical activity, smoking history, alcohol consumption, plasma blood sugar, ALT, HDL-C, LDL-C, and CRP levels. Independent variables significantly associated with HTN and CHD in chi-square (χ2) test were subjected to a multinomial logistic regression model to reveal independent predictors of HTN and CHD. Statistical significance was tested at 95% confidence interval (95% CI) (alpha ≤0.05).
Results
General characteristics of the study population
The study recruited 100 patients of which 31% and 69% were men and women, respectively.
More than three-quarter (81%) of the patients were within the age category of 45 years and above, while only 19% were below 45 years. Nearly half (45%) of all patients had attained primary education while only 9% had no formal education. More than two-third of the patients were married and 72% were self-employed. More than half (53%) of the patients had a family history of HTN and CHDs. Further, 65% of patients were clinically diagnosed with HTN, whereas 23% of patients were diagnosed with CHD and 12% had both conditions (Table 3).
Table 3
| Variables | Frequency (N=100) | Percentage (100%) |
|---|---|---|
| Gender | ||
| Men | 31 | 31 |
| Women | 69 | 69 |
| Age (years) | ||
| <45 | 19 | 19 |
| >45 | 81 | 81 |
| Education level | ||
| Higher education learning | 14 | 14 |
| Primary level | 45 | 45 |
| Secondary level | 32 | 32 |
| No formal education | 9 | 9 |
| Marital status | ||
| Married | 73 | 73 |
| No partner | 27 | 27 |
| Occupation | ||
| Formal-employment | 15 | 15 |
| Self-employed | 72 | 72 |
| Unemployed | 13 | 13 |
| Family history | ||
| Yes | 53 | 53 |
| No | 47 | 47 |
| Diseases type | ||
| HTN | 65 | 65 |
| CHD | 23 | 23 |
| HTN & CHD | 12 | 12 |
HTN, hypertension; CHD, coronary heart disease.
Proportions of lifestyles risk factors and biomarkers for HTN and CHDs
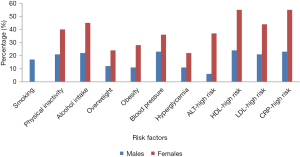
The proportion of lifestyle risk factors and biomarkers for HTN and CHDs were assessed for each patient involved in the study and results are summarized in Figure 1. Among the lifestyle risk factors assessed in this study, history of current alcohol (22% of males and 45% of females) consumption ranked the highest, followed by physical inactivity for female than male patients (40% versus 21%) (Figure 1). Similar observation for blood pressure (59%), overweight (36%) and obesity (39%) were recorded from these patients. High concentration of biomarkers for HTN and CHD were also recorded for female participants attending a cardiac clinic. It was observed that 55% of female patients had higher levels of HDL-C and CRP (>3 mmol/L), respectively. However, 24% of male patients were recorded to have abnormal HDL-C (0.90 to <1.15 mmol/L) and 23% of male patients had more than 3 mmol/L of CRP. Furthermore, more than one-third (37%) and 6% of female and male patients had higher ALT levels.
Association between socio-demographic characteristics, lifestyle and intermediate risk factors for HTN and CHDs
Socio-demographic characteristics and lifestyle, as well as studied intermediate risk factors, were associated with HTN and CHD (Table 4). In this study, gender, HTN, and CHD were significantly associated (P≤0.05). Likewise, age showed significant association with HTN and CHD (P≤0.001). No significant association (P≥0.05) was found between education level, occupation with HTN and CHD among patients.
Table 4
| Variables | Diseases type | χ2 test (P value) | ||
|---|---|---|---|---|
| HTN (N=65, 65%) | CHD (N=23, 23%) | HTN & CHD (N=12, 12%) | ||
| Socio-demographics | ||||
| Gender | 0.007* | |||
| Males | 22 | 2 | 7 | |
| Females | 43 | 21 | 5 | |
| Age (years) | 0.000* | |||
| <45 | 4 | 15 | 0 | |
| >45 | 61 | 8 | 12 | |
| Education level | 0.760 | |||
| HEL | 9 | 3 | 2 | |
| SEC | 20 | 8 | 4 | |
| PR | 28 | 12 | 5 | |
| NE | 8 | 0 | 1 | |
| Occupation | 0.614 | |||
| Formal employment | 9 | 4 | 2 | |
| Self-employed | 45 | 18 | 9 | |
| unemployed | 11 | 1 | 1 | |
| Lifestyle risk factors | ||||
| Physical activity | 0.350 | |||
| Yes | 28 | 6 | 5 | |
| No | 37 | 17 | 7 | |
| History smoking | 0.040* | |||
| Yes | 10 | 2 | 5 | |
| No | 55 | 21 | 7 | |
| Alcohol consumption | 0.764 | |||
| Yes | 45 | 14 | 8 | |
| No | 20 | 9 | 4 | |
| Body mass index (BMI) | 0.772 | |||
| 18.5–24.9 kg/m2 | 12 | 9 | 4 | |
| 25.0–29.9 kg/m2 | 25 | 7 | 4 | |
| ≥30.0 kg/m2 | 28 | 7 | 4 | |
| Blood pressure | 0.688 | |||
| Normal | 6 | 8 | 1 | |
| Pre-hypertension | 17 | 6 | 3 | |
| Hypertension stage I | 15 | 2 | 4 | |
| Hypertension stage II | 27 | 7 | 4 | |
| Biochemical markers | ||||
| Plasma glucose | 0.004* | |||
| Normal | 38 | 22 | 9 | |
| Hyperglycemia/diabetes | 27 | 1 | 3 | |
| ALT | 0.041* | |||
| Normal | 43 | 9 | 5 | |
| High risk | 22 | 14 | 7 | |
| LDL-C | 0.016* | |||
| Normal | 24 | 11 | 0 | |
| High risk | 41 | 12 | 12 | |
| HDL-C | 0.066 | |||
| Normal-males | 7 | 0 | 0 | |
| Normal-females | 7 | 6 | 1 | |
| Moderate-males | 8 | 2 | 4 | |
| Moderate-females | 23 | 10 | 3 | |
| High risk-males | 7 | 0 | 3 | |
| High risk-females | 13 | 5 | 1 | |
| CRP | 0.033* | |||
| Normal | 18 | 4 | 0 | |
| Moderate risk | 12 | 6 | 0 | |
| High risk | 35 | 13 | 12 | |
Chi square (χ2) test. Values were statistically significant (*P<0.05), HTN, hypertension; CHD, coronary heart diseases; ALT, alanine aminotransferase; CRP, C-reactive protein; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; HEL, Higher education learning; SEC, secondary level; PR, primary level; NE, no formal education.
With the exception of smoking history, which showed significant association with HTN and CHD (P≤0.05), physical activity, alcohol intake, blood pressure, and BMI were not associated with HTN and CHD (Table 4).
Moreover, blood plasma glucose, serum ALT, serum low-density lipoprotein cholesterol (LDL-C), and serum CRP were all significantly associated with HTN and CHD (P≤0.05) (Table 4).
Determination of independent predictors of HTN among patients
Results from Pearson’s chi-square (χ2) showed that gender, age, history of smoking, plasma blood glucose, ALT, low-density lipoprotein, and CRP levels were significantly associated with HTN. However, when subjected to multinomial logistic regression only age, ALT and glucose were independently associated with HTN risk (Table 5) and were retained.
Table 5
| Variable | Parameter estimated (B) | Standard error | P value | OR/Exp. (B) | 95% CI of OR |
|---|---|---|---|---|---|
| Age (years) | |||||
| <45 | −1.78 | 0.66 | 0.007 | 0.17 | 0.047–0.612 |
| >45 | Reference | – | – | – | – |
| ALT | |||||
| No risk | −1.18 | 0.49 | 0.018 | 3.24 | 1.22–8.57 |
| Risk | Reference | – | – | – | – |
| Glucose | |||||
| Normal | −1.53 | 0.64 | 0.016 | 0.22 | 0.62–0.76 |
| Hyperglycemia/diabetes | Reference | – | – | – | – |
ALT, alanine aminotransferase; CRP, C-reactive protein; CI, confidence interval; OR, odds ratio.
Nevertheless, the association between age and HTN was significant, where patients aged <45 years [odds ratio (OR) =0.17, 95% CI: 0.047–0.612] were at higher risks of HNT than those aged 45 years and above. Patients with normal ALT had a lower risk of HTN (OR =3.24, 95% CI: 1.22–8.57) than those with higher ALT levels. Further results showed a significant association between HTN and blood sugar, and patients with normal blood sugar (OR =0.22, 95% CI: 0.62–0.76) had reduced risk of HTN than diabetic patients.
Determination of independent predictors for CHD among patients
As shown in Table 6, the association between CHD risk and age were statistically significant (P=0.002). Patients aged <45 years were almost four times at high risk of suffering from CHD (OR =9.82, 95% CI: 2.37–40.62) compared to those aged >45 years. The risk for CHD was higher among patients with high ALT levels compared to those with normal ALT levels (OR =0.34, 95% CI: 0.12–0.93). Patients with high ALT levels were more likely to suffer from CHD, contrary to patients with normal blood sugar (OR =4.77, 95% CI: 1.31–17.42). Moreover, results showed that patients with normal CRP (OR =0.25, 95% CI: 0.08–0.79) had a lower risk for CHD compared to those with higher CRP levels.
Table 6
| Variable | Parameter estimated (B) | Standard error | P value | OR/Exp (B) | 95% CI of OR |
|---|---|---|---|---|---|
| Age (years) | |||||
| <45 | 2.28 | 0.72 | 0.002 | 9.82 | 2.37–40.62 |
| >45 | Reference | – | – | – | – |
| ALT | |||||
| No risk | −1.09 | 0.52 | 0.035 | 0.34 | 0.12–0.93 |
| Risk | Reference | – | – | – | – |
| Glucose | |||||
| Normal | −1.56 | 0.66 | 0.018 | 4.77 | 1.31–17.42 |
| Hyperglycemia/diabetes | Reference | – | – | – | – |
| CRP | |||||
| Normal | −1.38 | 0.58 | 0.018 | 0.25 | 0.08–0.79 |
| High level | Reference | – | – | – | – |
ALT, alanine aminotransferase; CRP, C-reactive protein; CHD, coronary heart disease; CI, confidence interval; OR, odds ratio.
Discussion
The study provides an overview of the assessment of CVD risk factors among diagnosed patients attending the cardiac clinic at a referral hospital in Tanzania. Findings showed a higher prevalence of HTN and CHD, with a higher proportion of patients being exposed to CVD risk factors despite the fact that they were under clinical management. Risk factors for these diseases were higher among female patients than male patients and this has been associated with higher number of female patients involved in this study 69% versus 31% for female and male, respectively. To date, there are only two studies population (28,29) conducted in Tanzania related to CHD and their associated risk factors. These studies have reported a lower prevalence of CHD and their associated risk, contrary to the current findings which showed a higher prevalence of CHD and their associated risk factors. This might be due to the fact that all patients who participated in this study had known medical conditions (HTN and CHD). Our study cannot rely on previous trends since these studies (28,29) were conducted in the past two decades. Results from this study reflect the current trend of CVDs, which is characterized by changes in lifestyle and epidemiological transition within Sub-Saharan countries (30). The current prevalence of HTN and CHD is higher compared to 37% and 9% prevalence of HTN and CHD, respectively (9).
This current study showed that 61% of patients did not engage in physical exercise for at least 30 minutes per week. Our findings, however, are in disagreement to that reported by the national STEP survey (31) and Ram and Trivedi (32) who found that 96.7% and 92.6% of study participants were physically active, respectively. Low level of physical activity among patients has been related to aging, cardiovascular symptoms and other chronic diseases, such as arthritis that reduces walking ability. The patients’ general health condition, shortage of breath, fatigue, and weakness have been reported as factors that lower levels of physical activity among patients with CHD (33,34). The percentage of physical inactivity observed among patients in this study were also inconsistent with other related study findings conducted by Stewart et al. (46%) (34), and Press et al. (17%) (33) among patients with CHD.
Regardless of the fact that all patients in this study were using drugs for the treatment of HTN and CHD, the majority (67%) of patients had a current history of alcohol intake. Patients with a history of alcohol intake were more affected by HTN (45%) and CHD (22%). This may be due to the key role played by alcohol in influencing atherosclerotic, inflammation and thrombosis formation that worsen the diseases condition (35). The overall prevalence of alcohol consumption was higher compared to that reported by Mbatia et al. (17.2%) (36), and by Francis et al. (20% and 22%) (37) in rural and urban areas of Tanzania, respectively. A lower level of knowledge on alcohol intake to the overall health status, reduction of drug efficacy and efficiency has been identified as factors that affect treatment progress among patients. This reduces the rate of recovery from the diseases and may result in more health complications such as heart failure, arterial fibrillation, and stroke (38).
In the present study, a large number of patients were overweight and obese, 36% and 39%, respectively. This has been related to the lower level of physical activity and poor eating habits, characterized by low consumption of fruits and vegetables. However, this was contrary to the findings from a European survey among patients with CHD, which found only 31% of the patients were obese (39). The prevalence of overweight and obesity found in this study is likely to worsen the health status of patients since it has been related to increased risk of CHD (39).
Moreover, the study found that majority of patients (59%) had uncontrolled blood pressure levels (systolic blood pressure ≥140 mmHg and diastolic blood pressure ≥90 mmHg), higher than 50% of the CHD patients who were found with higher levels of blood pressure in EUROASPIRE II Heart Survey Programme (39). However, this was almost similar with 57% of hypertensive participants (40) and lower compared to the prevalence of 37% reported in Dar es Salaam (41). The estimates of hypertensive people (10% to 20%) in the Sub-Saharan region (42) is lower compared to the current prevalence (59%). Higher levels of blood pressure among patients were related to higher consumption of dietary salts, which increases blood pressure. Dietary salt does not only increase blood pressure but also causes endothelial dysfunction, albuminuria, and development of kidney disease (43). World health organization recommend salt intake of <4 g/day among patients with high blood pressure (14). A diet low in sodium and increased potassium serves as a strategy to reduce blood pressure and decreases CVD mortality and morbidity (43). Study conducted in a hypertensive unit in Spain found that 42% of the CHD patients had attained a targeted goal of lowering blood pressure (<140 mmHg systolic and <90 mmHg diastolic) after management (44). Patients should be encouraged to reduce salt intake and dietary sodium and increases consumption of dietary potassium which can be achieved by eating legumes and a variety of fruits and vegetables (14). More education is thus needed in Tanzania when managing patients with HTN, especially on the importance of reducing salt intake, to meet the required target of blood pressure among the patients.
Diabetes is one of the important risk factors for the development of CVD (16). One-third of patients in the current study had uncontrolled plasma blood sugar, compared to 87% of the patients with higher (>6 mmol/L) plasma glucose reported in EUROASPIRE II Heart Survey Programme (39). The proportion of male (11%) and female (22%) patients with higher plasma glucose was lower compared to 15.3% of men and 23.2% of women reported from 14 clinical trials data (45). The overall prevalence of diabetes in the current study was higher compared to 21.7% reported in Kilimanjaro region by Stanifer et al. (46), and 5% and 2% found in urban and rural Tanzania, respectively (47). Patients with higher plasma glucose have also an increased risk of heart diseases and stroke due to insulin resistance (48).
Higher levels of ALT were recorded among study patients in this study. Higher levels of ALT are related to alcohol consumption, insulin resistance, obesity and other chronic diseases such as renal diseases (49). The present findings were similar with other reported in the cross-sectional analysis conducted in the United States (49), of which 267 CHD patients had >42 IU/L ALT levels. The present findings are contrary to 12% of patients found with higher ALT concentration in a study conducted in a primary care setting in central Virginia from 2010 to 2011 (50). Elevated ALT levels trigger the formation of atherosclerotic plaques, and it has been demonstrated that changes in lifestyle, as well as the use of lipid-lowering drugs, can return ALT levels to normal (51). The current study showed a significant association between higher ALT levels, HTN and CHD. Patients with a higher concentration of ALT had a higher risk for HTN and CHD than patients with normal ALT levels. Studies have found that patients with a higher concentration of ALT were more likely to be hypertensive and suffer from CHD than patients with normal ALT levels (52). High ALT levels were recognized as an independent predictor of CHD risk factors (49,53), similar to what was observed in the current study. Additionally, ALT has been associated with angiographic atherosclerosis score in women independently of metabolic syndrome and serum CRP concentration (51).
Higher CRP levels have been identified as a predictive marker for MI, stroke, and sudden cardiac death both health individual and in patients with stable CHD (22). CRP is produced in the presence of inflammation, infection and tissue damage (54). Uncontrolled HTN observed among patients has been related to increased CRP levels, as it causes damage to arteries (14). More than three-quarter of the study patients (78%) in this study were found with higher CRP levels, and the burden of HTN and CHD among these patients increased with increased CRP levels. Similar findings were reported in a Framingham Heart Study, which revealed 260 CHD patients with higher CRP levels (55). Similarly, the Hisayama Study (56) showed 129 CHD patients with >3 mg/L of CRP in the general population of Japanese. Higher levels of CRP have been associated with conventional risk factors for both ischemic vascular and non-vascular diseases (57). In our study, CRP was positively associated with CHD, this is similar with findings from the epidemiological data that showed an association between higher CRP levels and future CVD morbidity among those patients with known CHD (58). Other studies have also revealed the association between higher CRP levels and increased CHD events both among patients with known and unknown CHD (59,60). However, this was contrary to Casas et al. (61) who reported an insignificant association between CRP and CVD events including CHD.
The severity of CHD has been recognized to increase with aging (62), and it independently predicts CHD risk factors. In the current study, age was found to be an independent risk factor for CHD, and those patients aged <45 had higher CHD risk than elderly people, which was also contrary to other documented studies in developed countries, which showed decreased CHD mortality and morbidity among younger age groups (63). This might be affected by a small number of patients participated in the present study. Pal and Grera (64), also showed higher CHD risk factors among patients aged >50 years.
Results from this study also showed a significant association between plasma glucose, HNT, and CHD, which is similar with other study findings which showed a significant association between diabetes and risk factors for CVD, especially CHD (65). Additionally, current findings were similar to other reported studies which showed a significant association between diabetes and increased CVD risk factors (47,66).
This study had the following limitations; Limited time frame and financial resources that limited selection of a large number of participants. However, despite these constraints, obtained results from this study can still be used in the monitoring and evaluation of CVD disease management progression in other hospital settings in Tanzania.
Conclusions
This current study revealed a higher prevalence of both lifestyle and intermediate risk factors for HTN and CHD among clinically managed patients from a referral hospital in Tanzania. This indicates inadequate management of these risk factors among patients visiting the cardiac clinic. Poor management and monitoring of these risk factors can delay treatment outcome and cause more health complications, which will pose more health cost to the patients. These patients should be well educated on lifestyle modification, especially on healthy dietary habits and need for increased physical activity. Patients should also be well encouraged to reduce the amount of salt to the recommended levels (<5 g/day), for lowering blood pressure. This study further demonstrated the key role played by inflammatory markers particularly CRP and ALT in determining CVDs risk. This study highlights the need for further mechanic studies on determining how inflammatory markers (CRP and ALT) are involved with the pathogenesis of CHDs in this cohort of patients.
Acknowledgments
The authors wish to express their sincere gratitude to the management of KCMC for their cooperation during the study. Appreciation also goes to Mary Riwa for her assistance during data collection, Thadei Kavishe for his support during laboratory work, and Dr. Gloria Temu, for her assistance during field data collection.
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jxym.2019.03.05). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by Tanzania National Institute for Medical Research (NIMR) ethical committees (certificate number NIMR/HQ/R.8a/Vol.IX/2737), and informed consent was taken from all the patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- BeLue R. An overview of cardiovascular risk factor burden in sub-Saharan African countries: a socio-cultural perspective. Global Health 2009;5:10. [Crossref] [PubMed]
- WHO. Cardiovascular Disease: World Heart Day 2017. World Heal Organ, 2017.
- Roth GA, Johnson C, Abajobir A, et al. Global, Regional, and National Burden of Cardiovascular Diseases for 10 Causes, 1990 to 2015. J Am Coll Cardiol 2017;70:1-25. [Crossref] [PubMed]
- Gaziono TA, Bitton A, Anand S, et al. Growing Epidemic of Coronary Heart Disease in Low- and Middle- Income Countries. Currative Probl Cardiol 2011;35:72-115. [Crossref]
- Nkoke C, Luchuo EB. Coronary heart disease in sub-Saharan Africa: still rare, misdiagnosed or underdiagnosed? Cardiovasc Diagn Ther 2016;6:64-6. [PubMed]
- Ebireri J, Aderemi AV, Omoregbe N, et al. Interventions addressing risk factors of ischaemic heart disease in sub-Saharan Africa: a systematic review. BMJ Open 2016;6:e011881. [Crossref] [PubMed]
- Mensah GA. Ischaemic heart disease in Africa. Heart 2008;94:836-43. [Crossref] [PubMed]
- Peck RN, Green E, Mtabaji J, et al. Hypertension-related diseases as a common cause of hospital mortality in Tanzania: a 3-year prospective study. J Hypertens 2013;31:1806-11. [Crossref] [PubMed]
- Makubi A, Hage C, Lwakatare J, et al. Contemporary aetiology, clinical characteristics and prognosis of adults with heart failure observed in a tertiary hospital in Tanzania: the prospective Tanzania Heart Failure (TaHeF) study. Heart 2014;100:1235-41. [Crossref] [PubMed]
- World Health Organization. Global health risks: mortality and burden of disease attributable to selected major risks. Geneva: World Health Organization, 2009. Available online: https://apps.who.int/iris/handle/10665/44203
- WHO. A global brief on Hypertension: World Health Day 2013. World Heal Organ 2013;1-40.
- Reamy BV, Williams PM, Kuckel DP. Prevention of Cardiovascular Disease. Prim Care 2018;45:25-44. [Crossref] [PubMed]
- Olowe OA, Ross AJ. Knowledge, adherence and control among patients with hypertension attending a peri-urban primary health care clinic, KwaZulu-Natal. Afr J Prim Health Care Fam Med 2017;9:e1-7. [Crossref] [PubMed]
- Whitworth JAWorld Health Organization, International Society of Hypertension Writing Group. 2003 World Health Organization (WHO)/International Society of Hypertension (ISH) statement on management of hypertension. J Hypertens 2003;21:1983-92. [Crossref] [PubMed]
- Danaei G, Finucane MM, Lin JK, et al. National, regional, and global trends in systolic blood pressure since 1980: systematic analysis of health examination surveys and epidemiological studies with 786 country-years and 5·4 million participants. Lancet 2011;377:568-77. [Crossref] [PubMed]
- WHO. Global status report on noncommunicable diseases 2014. World Health Organization, 2014;176.
- National Heart Foundation of Australia. Guideline for the diagnosis and management of hypertension in adults - 2016. Melbourne: National Heart Foundation of Australia, 2016.
- Cléroux J, Feldman RD, Petrella RJ. Recommendations on physical exercise training. Canadian Medical Association. 1999:21-8. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1230336/pdf/cmaj_160_9_S21.pdf
- Haines A, Patterson D, Rayner M, et al. Prevention of cardiovascular disease. Occas Pap R Coll Gen Pract 1992;67-78. [PubMed]
- Neter JE, Stam BE, Kok FJ, et al. Influence of weight reduction on blood pressure: a meta-analysis of randomized controlled trials. Hypertension 2003;42:878-84. [Crossref] [PubMed]
- O'Donnell MJ, Mente A, Smyth A, et al. Salt intake and cardiovascular disease: why are the data inconsistent? Eur Heart J 2013;34:1034-40. [Crossref] [PubMed]
- Parikh SV, de Lemos JA. Biomarkers in cardiovascular disease: integrating pathophysiology into clinical practice. Am J Med Sci 2006;332:186-97. [Crossref] [PubMed]
- Wang J, Tan GJ, Han LN, et al. Novel biomarkers for cardiovascular risk prediction. J Geriatr Cardiol 2017;14:135-50. [PubMed]
- Ridker PM, Hennekens CH, Buring JE, et al. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N Engl J Med 2000;342:836-43. [Crossref] [PubMed]
- Mayige M, Kagaruki G. Tanzania Steps Survey Report. Ministry of Health and Social Welfare, and National Institute for Medical Research (NIMR) in Collaboration With World Health Organiztion, 2013. Available online: https://www.who.int/ncds/surveillance/steps/UR_Tanzania_2012_STEPS_Report.pdf
- Global Status Report on Noncommunicable Diseases 2014: Attaining the Nine Global Noncommunicable Diseases Targets; A Shared Responsibility. World Heal Organization, 2014. Available online: https://apps.who.int/iris/bitstream/handle/10665/148114/9789241564854_eng.pdf;jsessionid=EFB773FEBAA6DB2D9A6C25DAD3F0B633?sequence=1
- Chobanian AV, Bakris GL, Black HR, et al. Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension 2003;42:1206-52. [Crossref] [PubMed]
- Swai AB, McLarty DG, Kitange HM, et al. Low prevalence of risk factors for coronary heart disease in rural Tanzania. Int J Epidemiol 1993;22:651-9. [Crossref] [PubMed]
- Kitange HM, Swai AB, Masuki G, et al. Coronary heart disease risk factors in sub-Saharan Africa: studies in Tanzanian adolescents. J Epidemiol Community Health 1993;47:303-7. [Crossref] [PubMed]
- Kalage R, Blomstedt Y, Preet R, et al. INDEPTH Training and Research Centres of Excellence (INTREC). Tanzania Country Report October 2012;(October):1-81. Available online: http://www.intrec.info/Country%20reports/INTREC%20-%20Tanzania.pdf
- Mashili FL, Kagaruki GB, Mbatia J, et al. Physical Activity and Associated Socioeconomic Determinants in Rural and Urban Tanzania: Results from the 2012 WHO-STEPS Survey. Int J Popul Res 2018;2018:4965193. [Crossref]
- Ram RV, Trivedi AV. Behavioral risk factors of coronary artery disease: A paired matched case control study. J Cardiovasc Dis Res 2012;3:212-7. [Crossref] [PubMed]
- Press V, Freestone I, George CF. Physical activity: the evidence of benefit in the prevention of coronary heart disease. QJM 2003;96:245-51. [Crossref] [PubMed]
- Stewart R, Held C, Brown R, et al. Physical activity in patients with stable coronary heart disease: an international perspective. Eur Heart J 2013;34:3286-93. [Crossref] [PubMed]
- Mukamal KJ, Rimm EB. Alcohol's effects on the risk for coronary heart disease. Alcohol Res Health 2001;25:255-61. [PubMed]
- Mbatia J, Jenkins R, Singleton N, et al. Prevalence of alcohol consumption and hazardous drinking, tobacco and drug use in urban Tanzania, and their associated risk factors. Int J Environ Res Public Health 2009;6:1991-2006. [Crossref] [PubMed]
- Francis JM, Weiss HA, Mshana G, et al. The Epidemiology of Alcohol Use and Alcohol Use Disorders among Young People in Northern Tanzania. PLoS One 2015;10:e0140041. [Crossref] [PubMed]
- Conen D. Alcohol consumption and incident cardiovascular disease: not just one unifying hypothesis. Eur Heart J 2015;36:897-8. [Crossref] [PubMed]
- EUROASPIRE II Study Group. Lifestyle and risk factor management and use of drug therapies in coronary patients from 15 countries; principal results from EUROASPIRE II Euro Heart Survey Programme. Eur Heart J 2001;22:554-72. [Crossref] [PubMed]
- Njelekela MA, Mpembeni R, Muhihi A, et al. Gender-related differences in the prevalence of cardiovascular disease risk factors and their correlates in urban Tanzania. BMC Cardiovasc Disord 2009;9:30. [Crossref] [PubMed]
- Zack RM, Irema K, Kazonda P, et al. Determinants of high blood pressure and barriers to diagnosis and treatment in Dar es Salaam, Tanzania. J Hypertens 2016;34:2353-64. [Crossref] [PubMed]
- Reddy KS. Cardiovascular diseases in the developing countries: dimensions, determinants, dynamics and directions for public health action. Public Health Nutr 2002;5:231-7. [Crossref] [PubMed]
- Aaron KJ, Sanders PW. Role of dietary salt and potassium intake in cardiovascular health and disease: a review of the evidence. Mayo Clin Proc 2013;88:987-95. [Crossref] [PubMed]
- Banegas JR, Segura J, Ruilope LM, et al. Blood pressure control and physician management of hypertension in hospital hypertension units in Spain. Hypertension 2004;43:1338-44. [Crossref] [PubMed]
- Khot UN, Khot MB, Bajzer CT, et al. Prevalence of conventional risk factors in patients with coronary heart disease. JAMA 2003;290:898-904. [Crossref] [PubMed]
- Stanifer JW, Cleland CR, Makuka GJ, et al. Prevalence, Risk Factors, and Complications of Diabetes in the Kilimanjaro Region: A Population-Based Study from Tanzania. PLoS One 2016;11:e0164428. [Crossref] [PubMed]
- Aspray TJ, Mugusi F, Rashid S, et al. Rural and urban differences in diabetes prevalence in Tanzania: the role of obesity, physical inactivity and urban living. Trans R Soc Trop Med Hyg 2000;94:637-44. [Crossref] [PubMed]
- Choi JS, Kim JH, Ali MY, et al. Coptis chinensis alkaloids exert anti-adipogenic activity on 3T3-L1 adipocytes by downregulating C/EBP-α and PPAR-γ. Fitoterapia 2014;98:199-208. [Crossref] [PubMed]
- Ioannou GN, Weiss NS, Boyko EJ, et al. Elevated serum alanine aminotransferase activity and calculated risk of coronary heart disease in the United States. Hepatology 2006;43:1145-51. [Crossref] [PubMed]
- Siddiqui MS, Sterling RK, Luketic VA, et al. Association between high-normal levels of alanine aminotransferase and risk factors for atherogenesis. Gastroenterology 2013;145:1271-9.e1-3.
- Adibi P, Sadeghi M, Mahsa M, et al. Prediction of coronary atherosclerotic disease with liver transaminase level. Liver Int 2007;27:895-900. [Crossref] [PubMed]
- Goessling W, Massaro JM, Vasan RS, et al. Aminotransferase levels and 20-year risk of metabolic syndrome, diabetes, and cardiovascular disease. Gastroenterology 2008;135:1935-44, 1944.e1.
- Schindhelm RK, Dekker JM, Nijpels G, et al. Alanine aminotransferase predicts coronary heart disease events: a 10-year follow-up of the Hoorn Study. Atherosclerosis 2007;191:391-6. [Crossref] [PubMed]
- Casas JP, Shah T, Hingorani AD, et al. C-reactive protein and coronary heart disease: a critical review. J Intern Med 2008;264:295-314. [Crossref] [PubMed]
- Wilson PW, Nam BH, Pencina M, et al. C-reactive protein and risk of cardiovascular disease in men and women from the Framingham Heart Study. Arch Intern Med 2005;165:2473-8. [Crossref] [PubMed]
- Arima H, Kubo M, Yonemoto K, et al. High-sensitivity C-reactive protein and coronary heart disease in a general population of Japanese: the Hisayama study. Arterioscler Thromb Vasc Biol 2008;28:1385-91. [Crossref] [PubMed]
- Emerging Risk Factors Collaboration. C-reactive protein concentration and risk of coronary heart disease, stroke, and mortality: an individual participant meta-analysis. Lancet 2010;375:132-40. [Crossref] [PubMed]
- Zakynthinos E, Pappa N. Inflammatory biomarkers in coronary artery disease. J Cardiol 2009;53:317-33. [Crossref] [PubMed]
- Danesh J, Wheeler JG, Hirschfield GM, et al. C-reactive protein and other circulating markers of inflammation in the prediction of coronary heart disease. N Engl J Med 2004;350:1387-97. [Crossref] [PubMed]
- Ridker PM. Clinical application of C-reactive protein for cardiovascular disease detection and prevention. Circulation 2003;107:363-9. [Crossref] [PubMed]
- Casas JP, Shah T, Cooper J, et al. Insight into the nature of the CRP-coronary event association using Mendelian randomization. Int J Epidemiol 2006;35:922-31. [Crossref] [PubMed]
- Idris I, Deepa R, Fernando DJ, et al. Relation between age and coronary heart disease (CHD) risk in Asian Indian patients with diabetes: A cross-sectional and prospective cohort study. Diabetes Res Clin Pract 2008;81:243-9. [Crossref] [PubMed]
- Kotseva K, Wood D, De Bacquer D, et al. EUROASPIRE IV: A European Society of Cardiology survey on the lifestyle, risk factor and therapeutic management of coronary patients from 24 European countries. Eur J Prev Cardiol 2016;23:636-48. [Crossref] [PubMed]
- Pal RK, Grera A. Coronary Artery Disease in Africa: Community based study of Risk Factors. BJMP 2010;3:326.
- Chiwanga FS, Njelekela MA, Diamond MB, et al. Urban and rural prevalence of diabetes and pre-diabetes and risk factors associated with diabetes in Tanzania and Uganda. Glob Health Action 2016;9:31440. [Crossref] [PubMed]
- Ayah R, Joshi MD, Wanjiru R, et al. A population-based survey of prevalence of diabetes and correlates in an urban slum community in Nairobi, Kenya. BMC Public Health 2013;13:371. [Crossref] [PubMed]
Cite this article as: Roman WP, Martin HD, Sauli E. Assessment of risk factors for cardiovascular diseases among patients attending cardiac clinic at a referral hospital in Tanzania. J Xiangya Med 2019;4:18.


