
Functional assessment of achalasia
Introduction
Achalasia is a relatively rare disorder, with an estimated incidence of 1/100,000. In North America, its incidence is estimated to be 1.63/100,000, and its prevalence is 10.6/100,000 and rising, presumably due to its long disease duration, and survival (1). Ethnic, gender and socio-economic factors also affect its incidence, and manifestations (2). Despite its rarity, achalasia, because of its distinctive progressive symptoms and abnormalities, with progressive esophageal dilation, is the best described esophageal motor disorder. It was first described by Willis, with his well-known method of treatment of esophageal dilation with a flexible whalebone (3). In the early 1900’s with the discovery of radiography, barium was used as a contrast agent to study esophageal anatomy and function (4). Chevalier Jackson was the first US endoscopist to develop and use of rigid endoscopy to diagnose esophageal disorders and remove foreign bodies. In his first textbook on endoscopy, he refers to “Stark’s pill experiment” of placing a tablet with a rigid endoscope in the proximal esophagus and watching its progression into the stomach. Lack of such progression was indicative of esophageal obstruction and/or absent motility (5). The typical symptoms of achalasia with dysphagia and regurgitation are insidious in onset, and even after patients see a physician, the correct diagnosis and treatment are often delayed. In a recently published study from Germany, by Niebisch et al., of 563 patients with achalasia, it took a mean of two years for the correct diagnosis to be made, faster than the mean of 3 years to make the correct diagnosis 15 years ago (6). Once the diagnosis is made, assessment of esophageal function, and its impairment by the disease is an important determinant of treatment options, and surgical decision, as well as follow-up.
Symptom evaluation
Taking a careful history of the symptoms is an important first step in the assessment of this disorder. The Eckardt score is the most widely used measure (7). It assigns a score of 0 (no symptoms) to 3 (severe) to main achalasia symptoms: weight loss, dysphagia, chest pain and regurgitation. These are added for a maximal score of 12 (Table 1). The Eckardt score is easy to elicit and calculate, typically a score <3 indicates that the disease is in remission, while a score >3 suggests a need for treatment or retreatment. There are issues that create potential bias with this system (8), but in 2018 it remains the most widely score used to quantitate symptoms in pts with achalasia.
Table 1
| Diagnostic test | Description | Significance | ||||
|---|---|---|---|---|---|---|
| Symptom Assessment Eckardt Score | Score | Weight Loss (kg) | Dysphagia | Chest Pain | Regurgitation | Score <3= Remission |
| 0 | None | None | None | None | Score >3= Needs Intervention | |
| 1 | <5 | Occasional | Occasional | Occasional | ||
| 2 | 5–10 | Daily | Daily | Daily | ||
| 3 | >10 | Each Meal | Each Meal | Each meal | ||
| Timed Barium Esophagogram | Esophageal Height measurement (cm) after ingestion of 200cc of barium; barium height at 0, 1 and 5 mins | Normal 100% emptying at 2 mins; <3 cm height @ 5 mins suggests good response to therapy | ||||
| High Resolution Esophageal Manometry (HREM) | Type I achalasia: IRP >15 mmHg* and 100% failed peristalsis (DCI <100 mmHg-s-cm) | Type I Intermediate response to therapy | ||||
| Type II achalasia: IRP >15 mmHg* 100% failed peristalsis, and pan-esophageal pressurization with ≥20% of swallows | Type II Best response to therapy | |||||
| Type III achalasia: IRP >15 mmHg*, absent peristalsis, and spastic (DL<4.5 sec) contractions with DCI >450 mmHg-s-cm with 20% of swallows | Type III Worst response to therapy | |||||
| HREM + Impedance measurement of saline bolus transit | Impedance topography color plot height (cm) above EGJ at 5 minutes after upright ingestion of a 200 cc saline bolus | Correlates with timed barium esophogram | ||||
| HREM + Impedance topographic swallows with a calculated esophageal impedance integral (EII) | EII helps assess effect of therapy on esophageal function | |||||
| Functional Lumen Imaging Probe (FLIP) | The catheter is placed across the EGJ, and the Distensibility Index (EGJ-DI) is measured using impedance planimetry measure balloon diameter, with simultaneous measurements of the pressure needed for distention, using a step-wise balloon distention starting at 5 cc, up to a maximum of 70 cc. EGJ-DI (mm2/Hg) is calculated by dividing the narrowest EGJ cross-sectional area by the balloon pressure recorded at 50 and 60 cc distention volumes. | An EGJ-DI <2.8–2.9 provides supportive evidence for diagnosis of achalasia in pts with equivocal findings on HREM | ||||
*, actual normal values depend on manometric system used.
Barium esophagogram
Radiographic contrast studies remain an important part of the functional evaluation of pts with achalasia. The esophageal diameter, and anatomic appearance of the esophagus do help determine disease severity and duration. In most patients who have not been treated, the esophagus slowly and progressively dilates in diameter as the disease progresses. Interestingly, esophageal diameter does not seem to correlate well with symptoms or response to therapy (9). Several radiographic signs can help distinguish primary achalasia from the 2–4% of patients, with secondary achalasia, most commonly a malignancy (10). In the 1990’s Richter et al. from Cleveland Clinic helped standardize the timed barium, as a simple reproducible technique to assess esophageal function in patients with achalasia (11). The patient was administered 100–250 mL of low density barium suspension, as much as they could tolerate without regurgitation or aspiration. Radiographs were then taken at 1, 2 and 5 minutes. The height of the barium column from its meniscus to the distal esophagus “bird’s beak” typical of achalasia, is measured. The most helpful primary variable measured is the % decrease in height of the barium column at 5 minutes. Normals have 100% emptying of the barium column by 2 minutes. The test is simple to perform, and has been found to have good reproducibility, as well as day to day variability both in normal controls and patients with achalasia (12). The presence of esophageal stasis with slow emptying on a timed barium esophogram can help predict recurrent symptoms in patients with longstanding achalasia (13), although the predictive utility of this finding has been questioned recently (14). A timed 5-minute barium column height of less than 3 cm has been proposed in a recent study by the Northwestern group as indicative of a good response to therapy in patients with achalasia.
High resolution esophageal manometry
Esophageal motility was first evaluated by Code at Mayo Clinic in the 1950’s using a series of catheter placed esophageal balloons. Pope developed water perfusion manometric techniques in the 1960’s, which used small diameter catheters which were easy to customize and place. Using this system, reproducible manometric parameters of achalasia were developed, using 8–10 sensor perfused catheters with 4 lower esophageal sphincter (LES) placed sensors 90 degrees apart, or with a perfused Dent sleeve, and 4 sensors every 5 cm in the esophageal body to study peristaltic function. The diagnosis of achalasia required absent peristalsis using this system, and if present, lack of LES relaxation. These systems set the standard for more than 30 years, but provided limited data, and difficult accurate placement. In the early 2000’s, with increased computing power the development of solid state catheters, Clouse and the group from Washington University in St Louis pioneered the use of high resolution manometry, using catheters incorporating many more solid state sensors, and using color topography to be able to visually represent this large array of data. Pandolfino, Kahrilas and colleagues from Northwestern, as well as other research groups used this new High Resolution Esophageal Manometry (HREM) technique to develop, over a period of time, several new metrics to measure lower esophageal sphincter, and peristaltic function. This led to the Chicago classification that is currently in use in most laboratories, using an expert consensus type approach. This is now its in third iteration (version 3.0) (15). In Table 2 I have summarized the Chicago classification metrics of most interest to a clinician. These are the integrated relaxation pressure (IRP), the distal contractile integral (DCI) and the distal latency (DL). As the table indicates, the actual normal values depend on the specific manufacturer hardware used to collect the data. HREM along with the Chicago classification has become the primary diagnostic tool to evaluate esophageal function in patients with achalasia, as well as other motility disorders. This classification is evidence-based and will continue to be revised as more outcome data based on its use is generated.
Table 2
| HRM metric | Chicago definition (summary) | Clinical role |
|---|---|---|
| IRP (mmHg) | Mean of the 4 sec of maximal swallow-induced LES relaxation, 10 sec window after UES relaxation. Referenced to gastric pressure | An elevated IRP above normal defines lack of LES relaxation seen with achalasia, and is the first “branch point” of the Chicago classification |
| DCI (mmHg-s-cm) | Amplitude x duration x length (mmHg-s-cm) of the distal esophageal contraction | Measures strength of distal esophageal contraction |
| DL (sec) | Interval between UES relaxation and peristaltic contractile deceleration point <3 cm from proximal LES | Decreased DL (<4.5 sec) is an indicator of a spastic esophageal motor disorder such as type III achalasia |
IRP, integrated relaxation pressure; DCI, distal contractile integral; DL, distal latency.
The Chicago classification makes its initial branch point focused on the presence or absence of normal LES relaxation, with a latter sine qua non for diagnosing achalasia (12). Pandolfino et al. described three manometric phenotypes of achalasia: Type I—absent contractility; Type II—20% esophageal pan-pressurization, and Type III, so called “vigorous or spastic achalasia” with premature (DL <4.5 s) spastic contractions with a DCI of >450 mmHg-s-cm being present >20% of the time (16) (Table 1). His group also showed that theses phenotypes can help predict response to therapy, especially surgery, with the best response for the type II achalasia patients, and least response with the type III pts. Several other subsequent studies have confirmed this finding (17,18).
High resolution esophageal manometry with impedance (HREM-I)
This technology incorporates impedance sensors, which measure electrical conductivity of fluid combining with high resolution manometry, thus allowing us determine how well the esophagus clears ingested fluid. It is typically done using a saline solution. The Chicago group used the impedance data to generate new metrics which they felt would be potentially helpful in assessing response to treatment (19) It has been helpful in determining esophageal function and whether the esophagus clears the fluid ingested (20). Pandolfino and the Northwestern group developed a simple protocol modeled from that of the timed barium swallow. This was studied in 20 achalasia patients who were given a 200-cc saline challenge during Impedance HREM, with measurements of its height at 1 and 5 minutes. This was compared with the same measurements of timed barium swallow using 200 cc of barium. Using a barium or impedance height of >5cm as a definition of pathologic bolus retention, they found a 75% concordance at 1 min and 95% concordance in the 5min measurements between the two techniques (21). If reproduced by others this saline challenge protocol has the potential to simplify esophageal function testing in patients with achalasia, and eliminating the need for barium transit measurements. A recent German study corroborated the utility of using HREM-I to help separate achalasia from Esophago-gastric Junction (EGJ) outflow obstruction (22).
More recently the same group developed and esophageal impedance integral (EII), which uses the manometric software, to create a measurement box straddling the peristaltic pressure and impedance tracing, creating a swallow, and a post swallow impedance domains (20). It then uses a statistical software program (MATLab) to quantify the degree of the impedance bolus swallow that is not cleared by esophageal peristalsis. Normally 70% of swallows should be completely cleared from the esophagus. This determination is usually made qualitatively by the interpreting physician based on the color impedance heatmap generate with a swallow, and cleared by the peristaltic wave. In patients with achalasia where normal peristalsis is absent, the impedance integral allows a way to quantitate retention of each swallow bolus, to better assess the effect of therapy.
Functional lumen imaging probe (FLIP)
The newest diagnostic tool to help us functionally evaluate achalasia is FLIP technology (Figure 1). This uses a long high compliance balloon filled with saline. It uses 16 electrodes and a pressure sensor, and uses impedance planimetry technology to measure diameter as well as distensibility with a pressure volume curve of the esophageal sphincter and lumen. The EGJ distensibility index can be used to evaluate LES compliance and relaxation in patients with achalasia (24). This is especially useful in patients with borderline HREM metrics such as a high normal IRP or EGJ outflow obstruction, and typical radiographic findings of achalasia.
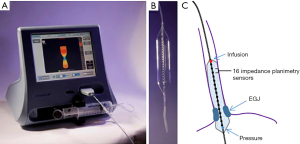
FLIP is also being investigated as tool to measure esophageal motility. When the esophagus is distended with the FLIP balloon it can generate secondary antegrade and retrograde wave patterns, not detectable with HREM. Pandolfino et al. used the FLIP—generated pressure patterns to show that there is residual motility activity in the esophagus in patients with achalasia (25). Recently these FLIP topographic patterns were compared by the Northwestern group with HREM findings in a group of 145 patients with dysphagia. A subset of patients with normal HREM had abnormal FLIP topography of the EGJ or esophageal body (26). The significance of these findings is not yet well understood, but there is a future potential evolving role for FLIP in the evaluation of patients with dysphagia and a variety of esophageal motor disorders, not just achalasia. FLIP measurements have also been used intra-operatively to help guide extent of POEM or Heller myotomy (27,28).
More recent developments in this area include development a more rigid type of balloon FLIP catheter, which can be used to only measure LES and stricture diameter, then dilate the esophagus. This balloon does not have pressure sensor, thus cannot measure an EGJ distensibility index. It however can accurately localize the LES or a stricture for dilation without fluoroscopy, and thus is a potentially new and helpful tool for achalasia therapy with forceful balloon dilation (29). The Clinical Practice Updates Committee of the American Gastroenterological Society (AGA) recently published an expert review of the current role of FLIP technology in the management of esophageal disorders. At present FLIP measurements have an adjunctive role, and complementary to other diagnostic methods, and should not be used alone to guide diagnosis or treatment (23).
Endoscopic ultra-sound (EUS)
Mittal et al. used an intra-luminal endoscopic catheter based ultrasound probe to show increased esophageal muscle thickness in patients with dysphagia and esophageal motor disorders (30). More recently Krishnan et al. used radial endosonography to evaluate 62 patients with esophageal motor disorders categorized according the Chicago Classification (31). EUS identified a previously overlooked anatomic cause for EGJ obstruction in 13% of patients, most commonly a malignancy. They found some differences in the median esophageal body and LES thickness in patients with an IRP >15, versus those with an IRP <15, but these were not striking. In patients with achalasia EUS has an adjunctive diagnostic role for excluding occult secondary causes of achalasia, but little role at this time in its functional assessment.
Conclusions
The functional assessment of achalasia remains an exciting area of research, with new and improved methods to help guide therapy. We now have more tools at our disposal to evaluate patients with dysphagia, but the modern HREM systems, are costly and not available in all hospitals. The Eckardt score and barium swallow, with barium transit measurement are inexpensive initial screening tests. Upper endoscopy is also helpful early on to exclude other causes. Symptomatic patients with suspected achalasia from these initial screening studies should be referred to centers with expertise in managing this disorder. Such centers need to have not only expertise in the needed diagnostic tools, such as HREM, but also a collaborative team of gastroenterologists and surgeons with the expertise to manage this complex disorder, and its complications, as well any complications of therapy. Recently the International Society for the Diseases of the Esophagus (ISDE) has published consensus guidelines for achalasia diagnosis and treatment (32). I have summarized in Table 3 their diagnostic recommendations, to summarize what is currently essential in the evaluation of this challenging disorder.
Table 3
| Test | Strength of recommendation | Expert agreement |
|---|---|---|
| HREM test of choice in the diagnosis of achalasia | Conditional recommendation. Low Grade | 94.2% |
| The Chicago Classification—helps define clinically relevant achalasia phenotypes | Good practice recommendation | 90.4% |
| Timed barium swallow is helpful in disease evaluation and treatment response | Conditional recommendation. Low Grade | 90% |
| Endoscopy should be performed in patients with achalasia to exclude malignancy | Good practice recommendation | 98.1% |
| The Eckardt score is helpful and should be used in the initial evaluation and follow-up of patients with achalasia | Good practice recommendation | 86.5% |
Acknowledgments
Funding: Walter&Lucille Rubin Foundation.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (David W. Rattner, Ozanan Meireles) for the series “Update on the Diagnosis and Treatment of Achalasia” published in Journal of Xiangya Medicine. The article has undergone external peer review.
Conflicts of Interest: The author has completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jxym.2019.03.02). The series “Update on the Diagnosis and Treatment of Achalasia” was commissioned by the editorial office without any funding or sponsorship. The author has no other conflicts of interest to declare.
Ethical Statement: The author is accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sadowski DC, Achah F, Jiang B, et al. Achalasia: incidence, prevalence and survival: a population based study. Neurogastroenterol Motil 2010;22:e256-61. [Crossref] [PubMed]
- van Hoeij FB. Incidence and costs of achalasia in The Netherlands. Neurogastroenterol Motil 2018;30. [PubMed]
- Fishchella PM, Jalilvand A, Lebenthal A. Diagnostic Evaluation of Achalasia: From the Whalebone to the Chicago Classification. World J Surg 2015;39:1593-7. [Crossref] [PubMed]
- Levine MS, Rubesin SE. History and Evolution of the Barium Swallow for Evaluation of the Pharynx and Esophagus. Dysphagia 2017;32:55-72. [Crossref] [PubMed]
- Jackson C. Tracheo-bronchoscopy, esohagoscopy and gastroscopy. St Louis The Laryngoscope Co 1907;15:123.
- Niebisch S, Hadzijusufovic E, Mehdorn M, et al. Achalasia-an unnecessary long way to diagnosis. Dis Esophagus 2017;30:1-6. [Crossref] [PubMed]
- Gockel I, Junginger T, Bernhard G, et al. Heller myotomy for failed pneumatic dilation: How effective? Ann Surg 2004;239:371-7. [Crossref] [PubMed]
- Taft TH, Carlson DA, Triggs J, et al. Evaluating the reliability and construct validity of the Eckardt symptom score as a measure of achalasia severity. Neurogastroenterol Motil. 2018;30:e13287. [Crossref] [PubMed]
- Martins P, Ferreira CS, Cunha-Melo JR, et al. Esophagus transit time in patients with chagasic megaesophagus. Lack of linear correlation between dysphagia and grade of dilation. Medicine (Baltimore) 2018;97:e0084. [Crossref] [PubMed]
- Gupta P, Debi U, Sinha SK, et al. Primary versus secondary achalasia: new signs of barium esophagogram. Indian J Radiol Imaging 2015;25:288-95. [Crossref] [PubMed]
- de Oliveira JM, Birgisson JM, Doinoff JM, et al. Timed barium swallow: A simple technique for evaluating esophageal emptying in patients with achalasia. AJR Am J Roentgenol 1997;169:473-9. [Crossref] [PubMed]
- Kostic S, Andersson M, Hellstrom M, et al. Timed barium esophagogram in the assessment of patients with achalasia: reproducibility and observer variation. Dis Esophagus 2005;18:96-103. [Crossref] [PubMed]
- Rohof WO, Lee A, Boeckxstaens GE. Esophageal stasis on a timed barium esophagogram predicts recurrent symptoms in patients with long-standing achalasia. Am J Gastroenterol 2013;108:49-55. [Crossref] [PubMed]
- van Hoeij. Smout AJ, Bredernoord AJ. Esophageal stasis in achalasia patients without symptoms after treatment does not predict symptom recurrence. Neurogastroenterol Motil 2017;29.
- Kahrilas PJ, Bredenoord AJ, Fox M, et al. The Chicago classification of esophageal motility disorders, v3.0. Neurogastroenterol Motil 2015;27:160-74. [Crossref] [PubMed]
- Pandolfino JE, Kwiatek MA, Nealis T, et al. Achalasia: A new clinically relevant classification by high resolution manometry. Gastroenterology 2008;135:1526-33. [Crossref] [PubMed]
- Salvador R, Constantini M, Zaninotto G, et al. The preoperative manometric pattern predicts the outcome of surgical treatment for esophageal achalasia. J Gastrointest Surg 2010;14:1635-45. [Crossref] [PubMed]
- Rohof WO, Salvator R, Annese V, et al. Outcomes of treatment of achalasia depend on manometric subtype. Gastroenterology 2013;144:718-25. [Crossref] [PubMed]
- Carlson DA, Lin Z, Kahrilas PJ, et al. High-Resolution Impedance Manometry Metrics of the Esophagogastric Junction for the Assessment of Treatment Response in Achalasia. Am J Gastroenterol 2016;111:1702-10. [Crossref] [PubMed]
- Carlson DA, Beveridge CA, Lin Z, et al. Improved assessment of bolus clearance in patients with achalasia using high resolution impedance manometry. Clin Gastroenterol Hepatol 2018;16:672-80.e1. [Crossref] [PubMed]
- Cho YK, Lipowaska AM, Nicodeme F, et al. Assessing bolus retention in achalasia using high resolution manometry with impedance: A comparator study with timed barium esophogram. Am J Gastroenterol 2014;109:829-35. [Crossref] [PubMed]
- Zizer E, Seufferlein T, Hänle MM. Impaired bolus clearance in combined high-resolution esophageal manometry and impedance measurement helps to differentiate between esophagogastric junction outflow obstruction and achalasia. Z Gastroenterol 2017;55:129-35. [Crossref] [PubMed]
- Hirano I, Pandolfino JE, Boeckstaens GE. Functional lumen imaging probe for the management of esophageal disorders: Expert review from the Clinical Practice Updates Committee of the AGA Institute. Clin Gastroenterol Hepatol 2017;15:325-34. [Crossref] [PubMed]
- Pandolfino JE, Ruich A DE, Nicodeme F, et al. Distensibility of the esophagogastric junction assessed with the functional lumen imaging probe (FLIP) in achalasia patients. Neurogastroenterol Motil 2013;25:496-501. [Crossref] [PubMed]
- Carlson DA, Lin Z, Kahrilas P, et al. The functional lumen imaging probe detects esophageal contractility not observed with manometry in patients with achalasia. Gastroenterology 2015;149:1742-51. [Crossref] [PubMed]
- Carlson DA, Kahrilas PJ, Lin Z, et al. Evaluation of esophageal motility utilizing the functional lumen imaging probe. Am J Gastroenterol 2016;111:1726-35. [Crossref] [PubMed]
- Teitelbaum EN, Soper NJ, Pandolfino JE, et al. An extended proximal esophageal myotomy is necessary to normalize EGJ distensibility during Heller myotomy for achalasia, but not POEM. Surg Endosc 2014;28:2840-7. [Crossref] [PubMed]
- Ngamruengphong S, von Randen BH, Filser J, et al. Intra-operative measurement of esophageal of esophagogastric junction cross-sectional area by impedance planimetry correlates with clinical outcomes of peroral endoscopic myotomy for achalasia: a multicenter study. Surg Endosc 2016;30:2886-94. [Crossref] [PubMed]
- Kappelle WF, Bogle A, Siersema PD. Hydraulic dilation with a shape-measuring balloon in idiopathic achalasia: a feasibility study. Endoscopy 2015;47:1028-34. [Crossref] [PubMed]
- Dogan I, Puckett JL, Padda PS, et al. Prevalence of increased esophageal muscle thickness in patients with esophageal symptoms. Am J Gastroenterol 2007;102:137-45. [Crossref] [PubMed]
- Krishnan K, Lin CY, Keswani R, et al. Endoscopic ultrasound as an adjunctive evaluation in patients with esophageal motor disorders subtyped by high-resolution manometry. Neurogastroenterol Motil 2014;26:1172-8. [Crossref] [PubMed]
- Zanninotto G, Bennett C, Boeckxstaens G, et al. The 2018 ISDE Achalasia Guidelines. Dis Esophagus 2018;31:1-29. [Crossref] [PubMed]
Cite this article as: Botoman VA. Functional assessment of achalasia. J Xiangya Med 2019;4:16.


