
Diagnosis of achalasia
Achalasia is a motility disorder defined as impaired relaxation in the esophagogastric junction (EGJ) and the esophageal body. The etiology has not been elucidated, while injury or loss of inhibitory neurons in the myenteric nerve plexus has been detected in some achalasia patients. The treatment of achalasia has been improved by a new endoscopic approach using a channelling endoscopic technique. Per oral endoscopic myotomy (POEM) is quickly becoming the mainstay of therapy for achalasia currently in most Asian countries due to its convenience and early recovery. The development of better diagnostics has increased our ability to diagnose achalasia and this may partly explain the fact that the incidence of achalasia has risen from 0.5–1.2 per 100,000 to 1.6 per 100,000 in some populations (1-3).
The diagnosis of achalasia depends on both an assessment of clinical symptoms and a careful structural and functional evaluation. The former includes mainly obtaining a history of progressive dysphagia and weight loss, while the latter includes the endoscopic, radiographic and manometric investigations. More recently, a new technique, endoscopic functional luminal imaging probe (EndoFLIP), is used to measure the distensibility in the EGJ in achalasia patients.
Clinical manifestation of achalasia
Dysphagia is the primary symptom of achalasia. Patients will show a progressive presentation. The patients would first have difficulty in swallowing solid food with some having a sensation of food impaction in the esophagus during the meal with the requirement of additional liquid intake to relieve the symptoms. Some patients may present with chest pain and this symptom has an unpredictable natural history. With the development of the disease, the dysphagia progresses, patients are unable to complete even liquid swallows and they may start to lose weight at this point due to avoidance of eating or due to frank regurgitation. Many patients are initially confused with GERD patients as regurgitation with or without heartburn is common when bolus retention is significant. In order to enhance the transit of the swallowed food, the achalasia patients would adjust their usual way in eating, some will try to swallow more slowly, or sometimes try to seek help with some maneuvers, including taking some liquid or arching the back.
The Eckardt score is a commonly used scoring system for achalasia (4). Weight loss, dysphagia, retrosternal pain and regurgitation are all captured in this scoring system with the highest score of 12. This score is commonly used for the evaluation of the severity of the achalasia symptom, and also be used as the tool to indicate the efficacy of treatment.
Endoscopic investigation
The diagnosis of achalasia cannot be confirmed by upper endoscopy; however, the endoscopic investigation is necessary to rule out alternative diagnoses and assess the anatomy. Dilation of the esophageal body can be observed during the procedure with lots of saliva or food retained in the esophageal lumen due to the distal obstruction caused by the relaxation dysfunction (Figure 1A). Obvious resistance would be felt when passing the endoscopy through the cardia. Normal mucosa is presented sometimes with edema in the cardiac area. Malignancy and other abnormality need to be excluded with upper endoscopy. Esophageal squamous cell carcinoma and esophageal adenocarcinoma are the most common malignancies in this patient population and careful examination should be done to exclude the mechanical esophageal obstruction. Other abnormality including the leiomyoma, esophageal ulcer, or eosinophil esophagitis.

Radiographic investigation
A typical appearance of barium esophagogram is “bird’s beak” in achalasia patients, this described the narrowing of the EGJ and dilation of the distal esophagus (Figure 1B). However, this “bird’s beak” sign was usually found in the case with confirmed achalasia, so abnormal esophagogram is not necessary to confirm the diagnosis of achalasia. The atypical appearance of achalasia in barium studies also includes the slow transit of barium through the gastroesophageal junction.
As a noninvasive method, time barium esophagram (TBE) objectively quantifies esophageal emptying and also the morphology. It has been reported by Kachala et al. that the change in the longitudinal muscle after the myotomy could predict the long-term efficacy of the surgery, and could identify those who need a second intervention (5). Thus, this could be a very good option for the esophageal function follow-up after the surgery (5).
Computed topography (CT) is not an ordinary test for achalasia. However, for patients with old age or atypical symptoms, or patients without the typical sign in barium radiography, CT scan is recommended to exclude the external compression, or if mucosal tumor found on upper endoscopy.
Endoscopic ultrasound (EUS)
The EUS is actually not a regular procedure for the diagnosis of achalasia. The EUS was firstly used in achalasia patients in 1989 by Deviere et al., in their study the thickness of the muscle layer of lower esophageal sphincter (LES) was measured (6). It was reported that the achalasia patients had much thicker LES than control group (6). However, this has been questioned by other investigators due to various results obtained from the application of EUS in the measurement of muscular layer thickness in achalasia patients (7). With the development of high frequency EUS probe, the identification of inner and outer muscular layer in the EGJ is feasible, thus make it possible to establish the difference of the muscular layer in the EGJ between the achalasia patients and healthy population. It was reposted by Barthet et al. that the fourth hypoechoic layer of the cardia wall was thicker in achalasia patients. However, the difference between them was very slight thus the EUS might not be a helpful tool to diagnose achalasia (8).
The other role of EUS in achalasia is to rule out pseudo-achalasia. The mass originated from the muscular layer of or outside EGJ could be detected by EUS. The inflammation in the muscular layer could also be detected. EUS has also been used to guide the therapy of achalasia including the Botox injection and the intra-operation evaluation during POEM.
Manometric investigation
Esophageal manometry has been recognized as the gold standard for the diagnosis of achalasia. Within the last decades, esophageal manometry has evolved from the water perfused to solid state manometry. Using the solid-state manometry, more manometry sensors were able to be integrated into one single manometry catheter which thus depicts the detailed pressure changes along the whole length of the esophagus, known as high resolution manometry (HRM). With HRM, the conventional line tracing of the esophageal manometric data was replaced by the esophageal pressure topography, which was firstly introduced by Ray Clouse. The color topography interprets the esophageal motility data with the intensity of the color, where the warmer the color indicate the higher pressure (Figure 2). Thus, the advantage of the HRM is to display the esophageal motility in a more visual way.

In the conventional line tracing manometry, it has been proposed by Dr. Spechler and Castell that the manometric features for a diagnosis of classic achalasia are: (I) incomplete relaxation of the LOS (defined as a mean swallow induced fall in resting LOS pressure to a nadir value >8 mm above gastric pressure) and (II) aperistalsis in the body of the esophagus characterized either by simultaneous esophageal contractions with amplitudes <40 mmHg or by no apparent esophageal contractions (9). Manometric features that are characteristic of classic achalasia but not required for the diagnosis include: (I) elevated resting LES pressure (>45 mmHg) and (II) resting pressure in the esophageal body that exceeds resting pressure in the stomach. A number of abnormalities in upper esophageal sphincter (UES) function also have been described in achalasia including: (I) elevated UES residual pressure, (II) decreased duration of UES relaxation, (III) repetitive UES contractions, and (IV) an abnormal belch reflex. These UES abnormalities are not the necessities required to establish the diagnosis of classic achalasia.
With the updated pressure topography in HRM, a new criterion of esophageal motility paralleled with the conventional manometry criteria was developed. The new criteria were named as Chicago classification where the first version of this classification was established. The Chicago classification was conceived by Dr. Kahrilas and his team of the basis of the HRM data of 400 patients and 75 normal controls (10). The new classification was widely adopted into clinical practice and research, and it has been redefined by the HRM Working Group in Chicago in 2014 (11). In the updated Chicago classification V3.0, achalasia has been divided into 3 subtypes (Table 1, Figure 3). It should be noted that an additional disorder of EGJ outflow obstruction other than achalasia is listed in the Chicago classification (11). This disorder does not meet the criteria of achalasia, while most of these patients have the common symptoms including dysphagia, chest pain and regurgitation. Patients with EGJ outflow obstruction are heterogeneous with unclear clinical significance, and a small portion of these patients would develop to achalasia at follow-up. It has been reported by Scherer et al. that 16 out of 40 EGJ outflow obstruction patients had mechanical obstruction, 8 patients had post-fundoplication dysphagia, while the other 16 patients were with idiopathic EGJ outflow obstruction who were akin to achalasia patients in either clinical phenotype or response to the management (12).
Table 1
| Subtypes | Esophageal pressure topography features |
|---|---|
| Type I achalasia (classic achalasia) | Elevated median IRP (>15 mmHg*), 100% failed peristalsis (DCI <100 mmHg-s-cm) |
| Type I achalasia (with esophageal compression) | Elevated median IRP (>15 mmHg*), 100% failed peristalsis, panesophageal pressurization with ≥20% of swallows |
| Type III achalasia (spastic achalasia) | Elevated median IRP (>15 mmHg*), no normal peristalsis, premature (spastic) contractions with DCI >450 mmHg-s-cm with ≥20% of swallows |
| EGJ outflow obstruction | Elevated median IRP (>15 mmHg*), sufficient evidence of peristalsis such that criteria for types I–III achalasia are not met |
*, cutoff value dependent on the manometric hardware. IRP, integrated relaxation pressure; DCI, distal contractile integral.
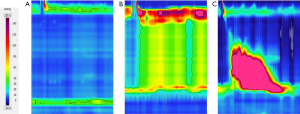
Different subtypes of achalasia under HRM distinguish each other in both the clinical presentation and also treatment outcome. It has been reported by the Northwestern team (13) that the degree of the esophageal lumen dilation was obviously different, and the treatment success rate in subtype II achalasia was more than 90%, which was the best among all subtypes. And multifactorial logistic regression indicates the both the degree of dilation in the esophageal lumen and the subtypes under HRM are independent predictive factors for the treatment outcome.
Endoscopic functional lumen imaging probe (EndoFLIP)
Decreased EGJ compliance and poor opening are characteristics of achalasia, thus it could be helpful to elucidate the intraluminal distensibility, and this could be obtained by applying the updated technology—endoscopic functional luminal imaging probe (EndoFLIP) (14) (Figure 4). The EndoFLIP system includes a computer-like work station and a catheter connected to the work station. There is a balloon fixed to the distal part of the catheter, catheter, 16 paired impedance planimetry sensors are installed into the catheter within the balloon section (Figure 4). The catheter connects to the work station with two connectors, one to the computer port of the work station, the other to the 80 mL syringe filled with conductive fluid which is controlled by the system and the conductive fluid could be continuously injected to the balloon mounted to the catheter. The transit of the conductive fluid on the impedance sensors along the balloon would generate the electric current, thus the cross-sectional area (CSA) is able to be measured by the impedance at a certain volume. There is also a pressure sensor installed at the distal part of the balloon which could provide the simultaneous intra-balloon pressure. The distensibility index of both the esophageal body and the EGJ are accessed by dividing the median narrowest CSA by the median intra-bag pressure over a set distension volume.
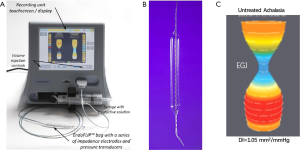
The decreased EGJ-DI was observed in the achalasia patients without treatment in some studies applying the EndoFLIP system, this indicated a narrower EGJ area when distending the balloon with higher pressure (15-17). What’s more, lower EGJ-DI was observed in achalasia patients with poor response to therapeutic intervention including pneumatic dilation and myotomy, while those with good response would show improved EGJ-DI (16,17); and more stronger association between EGJ-DI and symptom/esophageal retention under TBE was observed than that of manometric parameters since the EGJ-DI was better in characterizing the EGJ opening which was more important in determining the bolus retention than the EGJ relaxation defined by IRP in HRM. Besides, EndoFLIP might also provide additional information in the contractility in the esophageal body of achalasia patients which was not captured by HRM (18,19). Dr. Carlson has reported some specific contractile pattern in the esophageal body in achalasia patients including repetitive or retrograde pattern when using EndoFLIP to detect the esophageal body function in these patients. And these patterns were unique in different subtypes of achalasia defined by the HRM. The presence of these unique patterns might indicate the underlying various pathophysiology; thus, it might be helpful for further classifying the achalasia through their mechanism (19).
Conclusions
The diagnosis of achalasia should combine both clinical findings and auxiliary examination focused on structure and function. Endoscopic, radiographic and manometric investigations are necessary examinations for the diagnosis and exclusion of malignancy. Phenotypes defined by HRM are the predictors for the treatment outcome and may help inform treatment options. A newer modality, EndoFLIP, may provide a better understanding of esophageal body contractility and EGJ opening and may be a useful tool to explore the physiology of achalasia and assess treatment outcome.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (David W. Rattner, Ozanan Meireles) for the series “Update on the Diagnosis and Treatment of Achalasia” published in Journal of Xiangya Medicine. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jxym.2019.02.02). The series “Update on the Diagnosis and Treatment of Achalasia” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sadowski DC, Ackah F, Jiang B, et al. Achalasia: incidence, prevalence and survival. A population-based study. Neurogastroenterol Motil 2010;22:e256-61. [Crossref] [PubMed]
- Stein CM, Gelfand M, Taylor HG. Achalasia in Zimbabwean blacks. S Afr Med J 1985;67:261-2. [PubMed]
- O'Neill OM, Johnston BT, Coleman HG. Achalasia: a review of clinical diagnosis, epidemiology, treatment and outcomes. World J Gastroenterol 2013;19:5806-12. [Crossref] [PubMed]
- Eckardt VF. Clinical presentations and complications of achalasia. Gastrointest Endosc Clin N Am 2001;11:281-92. vi. [Crossref] [PubMed]
- Kachala SS, Rice TW, Baker ME, et al. Value of routine timed barium esophagram follow-up in achalasia after myotomy. J Thorac Cardiovasc Surg 2018;156:871-7.e2. [Crossref] [PubMed]
- Devière J, Dunham F, Rickaert F, et al. Endoscopic ultrasonography in achalasia. Gastroenterology 1989;96:1210-3. [Crossref] [PubMed]
- Ponsot P, Chaussade S, Palazzo L, et al. Endoscopic ultrasonography in achalasia. Gastroenterology 1990;98:253. [Crossref] [PubMed]
- Barthet M, Mambrini P, Audibert P, et al. Relationships between endosonographic appearan e and clinical or manometric features in patients with achalasia. Eur J Gastroenterol Hepatol 1998;10:559-64. [Crossref] [PubMed]
- Spechler SJ, Castell DO. Classification of oesophageal motility abnormalities. Gut 2001;49:145-51. [Crossref] [PubMed]
- Kahrilas PJ, Ghosh SK, Pandolfino JE. Esophageal motility disorders in terms of pressure topography: the Chicago Classification. J Clin Gastroenterol 2008;42:627-35. [Crossref] [PubMed]
- Kahrilas PJ, Bredenoord AJ, Fox MInternational High Resolution Manometry Working Group, et al. The Chicago Classification of esophageal motility disorders, v3.0. Neurogastroenterol Motil 2015;27:160-74. [Crossref] [PubMed]
- Scherer JR, Kwiatek MA, Soper NJ, et al. Functional esophagogastric junction obstruction with intact peristalsis: a heterogeneous syndrome sometimes akin to achalasia. J Gastrointest Surg 2009;13:2219-25. [Crossref] [PubMed]
- Pandolfino JE, Kwiatek MA, Nealis T, et al. Achalasia: a new clinically relevant classification by high-resolution manometry. Gastroenterology 2008;135:1526-33. [Crossref] [PubMed]
- Hirano I, Pandolfino JE, Boeckxstaens GE. Functional Lumen Imaging Probe for the Management of Esophageal Disorders: Expert Review From the Clinical Practice Updates Committee of the AGA Institute. Clin Gastroenterol Hepatol 2017;15:325-34. [Crossref] [PubMed]
- Smeets FG, Masclee AA, Keszthelyi D, et al. Esophagogastric junction distensibility in the management of achalasia patients: relation to treatment outcome. Neurogastroenterol Motil 2015;27:1495-503. [Crossref] [PubMed]
- Teitelbaum EN, Boris L, Arafat FO, et al. Comparison of esophagogastric junction distensibility changes during POEM and Heller myotomy using intraoperative FLIP. Surg Endosc 2013;27:4547-55. [Crossref] [PubMed]
- Rohof WO, Hirsch DP, Kessing BF, et al. Efficacy of treatment for patients with achalasia depends on the distensibility of the esophagogastric junction. Gastroenterology 2012;143:328-35. [Crossref] [PubMed]
- Carlson DA, Lin Z, Rogers MC, et al. Utilizing functional lumen imaging probe topography to evaluate esophageal contractility during volumetric distention: a pilot study. Neurogastroenterol Motil 2015;27:981-9. [Crossref] [PubMed]
- Carlson DA, Lin Z, Kahrilas PJ, et al. The Functional Lumen Imaging Probe Detects Esophageal Contractility Not Observed With Manometry in Patients With Achalasia. Gastroenterology 2015;149:1742-51. [Crossref] [PubMed]
Cite this article as: Xiao Y, Pandolfino JE. Diagnosis of achalasia. J Xiangya Med 2019;4:14.


