Laparoscopic Heller myotomy—A review of the literature
Introduction
Achalasia is a rare motility disorder of the esophagus which occurs as a result of decreased or absent inhibitory ganglion cells in the myenteric plexus of the esophagus and lower esophageal sphincter (LES) (1-3). The loss of these neurons is thought to be caused by inflammation related to an underlying autoimmune, viral or degenerative process (3). This results in the inability of the LES to relax as well as dysmotility of the esophageal body. Patients typically present with symptoms of dysphagia, regurgitation, chest pain and weight loss. Individuals with achalasia may also report heartburn, emesis, food avoidance and recurrent aspiration events or pneumonia (1-3). Treatment focuses on incapacitating the LES to decrease resistance for passage of the food bolus.
Workup
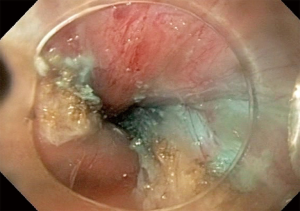
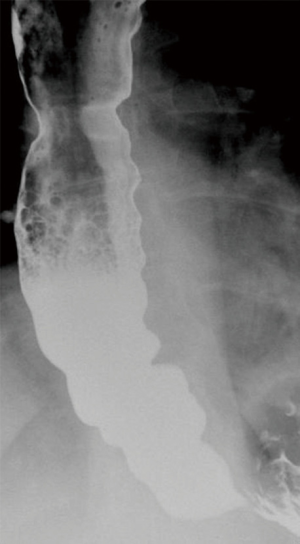
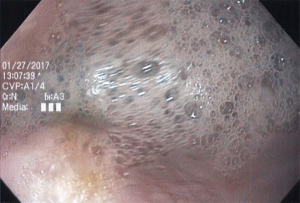
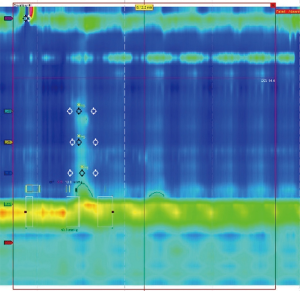
The workup of a patient presenting with symptoms consistent with achalasia should start with a barium swallow (3). This demonstrates a dilated esophagus with a distal taper or ‘bird-beak’ appearance (Figure 1) at the gastroesophageal junction (GEJ) (1,3). In severe cases, a tortuous esophagus may be present or there may be a concurrent epiphrenic diverticula (3). Additional work-up should include an endoscopy to rule out anatomical lesions concerning for pseudoachalasia. Classic endoscopic findings include retained food and secretions (Figure 2), a dilated or tortuous esophagus and subjectively increased pressure while passing through the GEJ (1,3). The most sensitive test for achalasia is esophageal manometry (3). While water perfusion catheters are still in use, a high-resolution esophageal manometry (HREM) allows for a more sensitive evaluation of esophageal peristalsis. Pathognomonic HREM findings of achalasia include dysmotility of the esophageal body and incomplete relaxation of the LES (Figure 3). The Chicago classification has given rise to three distinct classifications of achalasia which vary based on the quality of dysmotility proximal to the LES (1,3). All forms of achalasia are characterized by a lack of relaxation of the LES. While all forms of achalasia are treated similarly, they do have different response rates to intervention.
Treatment
Treatment of achalasia falls into one of three categories: pharmacologic, endoscopic, and surgical. Pharmacologic treatments include nitrates and calcium-channel blockers (1,3). Both of these medications act on the LES to induce relaxation. Manometric studies have shown that a reduction in LES resting pressure of 30–60% can be achieved (3,4). However, symptom improvement is marginal, and this strategy is largely ineffective in the long-term treatment due to tachyphylaxis. Further, both medications carry a significant side effect profile which makes use challenging or impossible (1,3). A more promising pharmacologic intervention is direct injection of botulinum toxin A into the LES. Studies indicate that 80–90% of patients experience resolution of symptoms at 1 month (3,5). Consistent with other indications for Botox, there is treatment fatigue with improvement typically only observed in 35–40% of patients at 1 year (3,5).
Pneumatic dilation
Pneumatic dilation was once considered the gold standard treatment of achalasia (3). The procedure involves placing a rigid balloon across the LES with a forceful disruption these muscle fibers under fluoroscopic guidance (2,3). Serial dilations are often necessary with excellent symptom relief achieved in up to 90% of patients (2,3,6). Despite good early results, a large number of patients will experience recurrent symptoms within 5 years (3,6,7). Risk factors for symptom recurrence include young age, male sex, classic achalasia, elevated LES pressure at 3 months and incomplete balloon expansion (3). Following pneumatic dilation patients may experience chest pain, fever or esophageal hematoma. The risk of esophageal perforation is typically quoted as being 1–2% with this procedure (3,6). Given the necessity of serial interventions and the durability and safety profile of a surgical myotomy, balloon dilation has fallen out of favor in many esophageal centers (2,3,6,8).
Per oral endoscopic myotomy (POEM)
The most recent innovation in the treatment of achalasia is POEM. Using a standard endoscope, the surgeon is able to incise the mucosa and submucosa to enter the submucosal space. A long submucosal tunnel is then created followed by an endoscopic myotomy (Figure 4). The mucosal defect is then closed either with clips or endoscopic suturing (2). To date, the efficacy of a POEM relative to a laparoscopic myotomy has been excellent with success rates quoted between 90–100% (3,9-11). The remaining concern with POEM is related to the risk of postoperative gastroesophageal reflux disease (GERD). Proponents of the POEM cite the persistence of the angle of His and lack of a hiatal dissection as protective against GERD after a POEM. The divergent argument is that the obliteration of the LES places the patient at a significant risk for reflux and obligates the patient to long term proton pump inhibitor use. Long term studies must be completed to objectively assess the presence and effect of pathologic reflux within the esophagus and assess for the durability of an endoscopic myotomy.
Heller myotomy
The Heller myotomy was first described in 1913 by Ernest Heller. Heller performed both anterior and posterior myotomies as a treatment of achalasia. The first minimally invasive Heller myotomy was performed thoracoscopically in 1991 and a description of a laparoscopic approach followed shortly after. The laparoscopic technique was found to allow for excellent visualization of the lower esophagus facilitating an anterior esophagomyotomy which could extend onto the cardia of the stomach while also allowing for the straightforward creation of an antireflux procedure (2). In 1997, Hunter et al. demonstrated a greater than 90% success rate with laparoscopic Heller myotomy (LHM) and fundoplication (12,13). Further, a study by Patti et al. in 1999 compared 168 patients who underwent thoracoscopic myotomy or LHM with fundoplication (3,12,14). They demonstrated that the patients who had a laparoscopic procedure with a fundoplication had significantly decreased incidence of GERD, shorter hospital stay and greater symptom relief (14). In addition, larger series have shown that the mortality of the procedure is less than 0.1% and that the rate of the most common complication (esophageal or gastric perforation) is low and almost always immediately recognized and repaired leading to a very low clinical impact (12). These reasons have led to the LHM being considered the gold standard surgical approach to achalasia.
Procedural steps
The steps to performing an LHM have been described in multiple sources (15,16). The reported methods are similar with most differences based on surgeon preference. The patient is positioned supine with arms out. A footboard is placed to allow for steep reverse Trendelenburg. The camera port is placed above the umbilicus to the left of midline through the rectus (15). Placement of this camera should allow for visualization of the hiatus and mediastinum. A trocar is placed in the midclavicular lines bilaterally for the surgeon. An assistant port is placed lateral to the left anterior axillary line. The left lobe of the liver is retracted through a subxiphoid or right anterior axillary line port. Alternate port orientations have been suggested with the general principle of allowing unhindered access to the hiatus and the mediastinum (15).
Access to the right crus is gained by dividing the pars flaccida cephalad towards the right crus. The phrenoesophageal ligament is then dissected off the esophagus avoiding injury to the anterior vagus nerve. After identifying the left crural pillar, dissection is carried posteriorly to the crural confluence. During this process it is often necessary to transect the short gastrics and medialize the stomach for adequate visualization of the more posterior proximal short gastric vessels. Finally, a retroesophageal window is developed with careful attention to not damage the posterior vagus nerve. Dissection of the retroesophageal window can then be continued from the right until a complete window is created to allow for the passage of Penrose drain to aid in downward traction of the stomach and esophagus (15). Dissection is then continued into the mediastinum to allow exposure for a long myotomy.
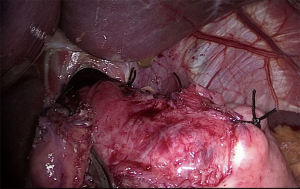
With the esophagus exposed the anterior vagus nerve is mobilized with the epiphrenic fat pad to allow for a long continuous myotomy while avoiding injury to the nerve. The course of the myotomy is often marked with cautery to avoid spiraling. The muscle is then incised starting on the esophagus above the GE junction. This incision has been described with laparoscopic scissors, hook cautery, laparoscopic energy devices and blunt dissection (15,16). Once the mucosa is identified a myotomy is performed up into the mediastinum. Visualization is facilitated with firm caudal and lateral retraction on the edges of the myotomy. Bleeding from the muscle edge is controlled with pressure using epinephrine soaked cherry dissectors, energy or suture (15,16). The myotomy is continued at least 5 cm above the GEJ and no less than 2 cm on to the gastric wall. This is typically the area of greatest technical difficulty and potential perforation. If a perforation is encountered this is dealt with by intracorporeal suture repair (15). Once the myotomy is complete, confirmation of complete myotomy and dynamic leak testing are performed with intraoperative endoscopy (15). The ability to maintain a view across the GEJ without active insufflation is an excellent marker for a complete myotomy (15). Alternatively, intraoperative manometry technology may be used to grade the quality of the myotomy. The procedure is concluded by performing an anti-reflux procedure (Figure 5). While there is controversy over which type of partial fundoplication is superior, the literature is clear that a 360-degree wrap is contraindicated due to the risk of post-operative dysphagia (12,15).
Heller myotomy compared to pneumatic dilation (PD)
Pneumatic dilation had long been considered the gold standard treatment for achalasia. Prior to the introduction of minimally invasive surgery, the morbidity of an open operation was unacceptably high given the efficacy and safety of PD. However, the LHM provides for significantly decreased morbidity and time for convalescence with similar functional outcomes when compared to an open approach. What’s more, there is increasing evidence that the LHM is superior to PD as well. Campos et al. in 2009 completed a review of case series comparing LHM with PD (8). They found that in patients undergoing PD improvement rates were quoted as being 68% compared to 89% in the LHM group (8). A large retrospective study performed by Lopushinsky and Urbach in 2006 looked at 1461 adults who underwent achalasia treatment between 1991 and 2002 (17). They noted that, in those who initially underwent PD, 36.8%, 56.2% and 63.5% of patients needed subsequent treatments at 1, 5, and 10 years respectively. Conversely, only 16.4%, 30.3% and 37.5% needed subsequent treatments if their index treatment was a LHM (17). A meta-analysis was performed by Illés et al. in 2017 examining LHM vs. PD found. Due to heterogeneity they analyzed studies based on a result of a “successful outcome” as defined by the author in each individual study. After analysis they found that significantly higher rates of successful outcomes were found after LHM compared to PD (7). The European Achalasia trial recently released their 5 year follow up data in which 201 patients were randomized to undergo LHM or PD (6). They did not find a significant difference between LHM and PD. However, 25% of patients who underwent PD required additional dilations with a 2% risk of esophageal perforation with each PD (6). Certainly, PD is an effective modality for addressing the sequelae of a hypertensive LES. However, the treatment often necessitates serial procedures with each intervention posing a risk of perforation. In contrast, a LHM is a safe treatment with lower need for reinterventions (17). It is these qualities that have led to the increased emphasis on a surgical approach to the treatment of achalasia.
Heller myotomy compared to POEM
Given that POEM is a relatively new procedure, long term data is still being collected. To date no long-term trial comparing POEM to LHM has been completed. Several meta-analyses have been performed which conclude that POEM and LHM have comparable short-term improvement in symptoms (3,9,10,12). One recent meta-analysis by Schlottman et al. reported a statistically significant greater improvement in symptoms after POEM compared to LHM in the short term (9). This finding may be attributed to fact that a POEM allows for a longer myotomy than would be feasible from an abdominal approach. In the setting of spastic achalasia this has been hypothesized to result in better symptom resolution. Supporting this would be the findings of Kumbhari et al. [2015] who examined POEM vs. LHM in patients who had type III achalasia (10). In this study, the authors concluded that a longer myotomy in those treated with a POEM resulted in statistically significant symptom improvement when compared to LHM group. Schlottmann et al. did note that, while their findings showed a statistically significant improvement with POEM, this equated to only a 5.5% absolute difference and caution was advised regarding drawing conclusions of POEM’s superiority (9).
The efficacy of POEM has been consistently strong in the reported literature (11). That is not to say that it has been universally adopted. Opponents of the technique cite an unacceptably high rate of pathologic GERD post procedure. The rate of reflux after a LHM is variable in the literature due to heterogeneity in assessing for this disease. A recent meta-analysis noted a pooled rate of pH proven reflux at 16.8% for LHM with a fundoplication versus 39% for those who had undergone a POEM (9). Another group reported an average DeMeester score of 39.7 at a mean follow-up of 7.6 months after POEM (18). Conversely, Chan et al. noted improved GERD-related outcomes after POEM and Ujiki identified no difference in outcomes based on operative approach (19,20). It is important to note that subjective symptoms are poor indicators of pathologic GERD in an achalasia population. Further, given the necessity of proton pump inhibitor treatment to suppress acid and the accumulating body of evidence that these medicines may have long term sequelae, this question will need to be addressed with objective long-term studies utilizing objective metrics to evaluate GERD moving forward (21).
Laparoscopic Heller myotomy and fundoplication
The question of whether a fundoplication is required has long been debated and studied in the literature. A randomized controlled trial (RCT) in 2003 demonstrated that patients who underwent LHM without fundoplication were significantly more likely to develop pathologic esophagitis in the post-operative period (22). In 2004, Richards et al. completed an investigation comparing LHM with LHM and anterior Dor fundoplication (23). This double-blind RCT randomized 43 patients and studied them at 6 months with manometry and 24-hour pH monitoring. Their results found pathological GERD in 47.6% of patients after LHM alone compared to 9.1% of patients after LHM and Dor fundoplication (23). No significant difference in LES pressure or postoperative dysphagia scores were observed between the groups. The same group released long term data of this same cohort using subjective scores of dysphagia and GERD. While they found that patients with LHM alone had a trend towards higher dysphagia and GERD scores this was not statistically significant (24). Despite the long-term findings, the results of these studies and of multiple observational studies have resulted in the current recommendation being to perform a fundoplication following a surgical myotomy (18).
The concern regarding pathological reflux risk after a LHM has prompted some surgeons to advocate for a full 360 degree wrap as would be performed as a part of an anti-reflux procedure for GERD. Rebecchi et al. [2008] completed an RCT looking at 144 patients who were randomized to total or partial fundoplication post LHM (25). At 5 years postoperatively dysphagia was significantly more frequent in the total fundoplication group (15%) compared to the partial fundoplication group (2.8%). Others have reported satisfactory results with a full wrap. However, given the adequacy of symptom control, improved side effect profile and the decreased risk of dysphagia, the general consensus is that partial wrap is the best option in this population.
The question remains as to whether an anterior or posterior wrap is superior. Proponents of the posterior fundoplication argue that this wrap provides better reflux control and allows for the wrap to be sutured to the edges of the myotomy keeping it open and preventing fibrosis of the myotomy edges. Conversely, proponents of the anterior fundoplication trumpet its relative technical ease and the advantage that it covers the exposed mucosa of the myotomy. In 2017 Torres-Villalobos et al. released results of a long-term RCT evaluating Dor versus Toupet fundoplication by HREM, pH testing and symptom scores (26). They found no significant difference in IRP or LES pressures at 6 and 24 months or abnormal acid exposure at 12 or 24 months between procedures. Additionally, no differences in symptom scores were noted at 1, 6 or 24 months between procedures (26). Similar findings were reported by Rawlings et al. in their group of 60 patients with no difference being detected in functional outcome or in 24-hour pH monitoring when comparing partial anterior and posterior fundoplication (27). The Dor fundoplication group did show a trend towards more postoperative acid reflux but this was not statistically significant. There is no clear evidence as to which type of fundoplication is superior. Conversely, the consensus remains that a partial fundoplication allows for similar symptom control with decreased risk of side effects when compared to a full wrap.
Recent work has discussed the technical variation of performing a LHM with limited hiatal dissection as an alternative to LHM with fundoplication. Several studies have been performed examining this with the premise being that if dissection can be performed by mobilizing only the anterior esophagus that the posterior and lateral esophageal attachments and antireflux barriers can be preserved (28,29). Early results have shown promise with this technique; however, the studies are small and lack long term data (28,29). Concern regarding the ability to perform a long and safe myotomy without complete esophageal mobilization has also been raised. At this point the standard is still to complete a partial fundoplication after myotomy.
Conclusions
Achalasia is a rare, chronic esophageal motility disorder. The treatment paradigm has shifted over the last century from a non-operative approach to one that is more interventional in nature. The most effective current surgical treatment for the disorder is a laparoscopic Heller myotomy. Recent evidence demonstrating the effectiveness of pneumatic dilation in experienced hands as well as the early promising results of POEM will lead to ongoing discussions regarding the best treatment options. It is likely that all treatment modalities will continue to be offered based on the patient’s presenting comorbidities, constellation of symptoms and risk preference. Long term studies with both subjective and objective evaluations of esophageal emptying and pathologic GERD will be critical to better characterize the indications and risks of each approach. With that said, the effectiveness of the LHM and its impressive safety profile continues to be demonstrated in the literature (30-32). Given the concerns regarding the potential issues related to long term PPI use (21) and the excellent reflux-related outcomes of a LHM, it will remain a mainstay in the treatment of achalasia for the foreseeable future.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (David W. Rattner, Ozanan Meireles) for the series “Update on the Diagnosis and Treatment of Achalasia” published in Journal of Xiangya Medicine. The article has undergone external peer review.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jxym.2019.02.03). The series “Update on the Diagnosis and Treatment of Achalasia” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Beck WC, Sharp K. Achalasia. Surg Clin North Am 2011;91:1031-7. [Crossref] [PubMed]
- Allaix ME, Patti M. Endoscopic dilation, heller myotomy, and peroral endoscopic myotomy: treatment modalities for achalasia. Surg Clin North Am 2015;95:567-578. [Crossref] [PubMed]
- Boeckxstaens GE, Zaninotto G, Richter J. Achalasia. Lancet 2014;383:83-93. [Crossref] [PubMed]
- Traube M, Dubovik S, Lange RC, et al. The role of nifedipine therapy in achalasia: results of a randomized, double-blind placebo-controlled study. Am J Gastroenterol 1989;84:1259-62. [PubMed]
- Leyden JE, Moss AC, Macmathuna P. Endoscopic pneumatic dilation versus botulinum toxin injection in the management of primary achalasia. Cochrane Database Syst Rev 2006;CD005046. [PubMed]
- Moonen A, Annese V, Belmans A, et al. Long-term results of the european achalasia trial: a multicenter randomized controlled trial comparing pneumatic dilation versus laparoscopic heller myotomy. Gut 2016;65:732-9. [Crossref] [PubMed]
- Illés A, Farkas N, Hegyi P. Is heller myotomy better than balloon dilation? A meta-analysis. J Gastrointestin Liver Dis 2017;26:121-7. [PubMed]
- Campos GM, Vittinghoff E, Rabl C, et al. Endoscopic and surgical treatments for achalasia: a systematic review and meta-analysis. Ann Surg 2009;249:45-57. [Crossref] [PubMed]
- Schlottmann F, Luckett DJ, Fine J, et al. Laparoscopic heller myotomy versus peroral endoscopic myotomy (POEM) for achalasia: a systematic review and meta-analysis. Ann Surg 2018;267:451-60. [Crossref] [PubMed]
- Kumbhari V, Tieu AH, Onimaru M, et al. Peroral endoscopic myotomy (POEM) vs laparoscopic heller myotomy (lhm) for the treatment of type III achalasia in 75 patients: a multicenter comparative study. Endosc Int Open 2015;3:E195-201. [Crossref] [PubMed]
- Swanstrom LL, Kurian A, Dunst CM, et al. Long-term outcomes of an endoscopic myotomy for achalasia: the poem procedure. Ann Surg 2012;256:659-67. [Crossref] [PubMed]
- Rebecchi F, Allaix ME, Schlottmann F, et al. Laparoscopic heller myotomy and fundoplication: what is the evidence? Am Surg 2018;84:481-8. [PubMed]
- Hunter JG, Trus T, Branum G, et al. Laparoscopic heller myotomy and fundoplication for achalasia. Ann Surg 1997;225:655-64. [Crossref] [PubMed]
- Patti MG, Pellegrini CA, Horgan S, et al. Minimally invasive surgery for achalasia: an 8-year experience with 168 patients. Ann Surg 1999;230:587-93. [Crossref] [PubMed]
- Nau P, Rattner D. Laparoscopic heller myotomy as the gold standard for treatment of achalasia. J Gastrointest Surg 2014;18:2201-7. [Crossref] [PubMed]
- Valverde A, Cahais J, Goasguen N, et al. Laparoscopic heller myotomy. J Visc Surg 2018;155:59-64. [Crossref] [PubMed]
- Lopushinsky SR, Urbach DR. Pneumatic dilation and surgical myotomy for achalasia. JAMA 2006;296:2227-33. [Crossref] [PubMed]
- Familiari P, Greco S, Gigante G, et al. Gastroesophageal reflux disease after peroral endoscopic myotomy: analysis of clinical, procedural and functional factors, associated with gastroesophageal reflux disease and esophagitis. Dig Endosc 2016;28:33-41. [Crossref] [PubMed]
- Chan SM, Wu JC, Teoh AY, et al. Comparison of early outcomes and quality of life after laparoscopic Heller’s cardiomyotomy to peroral endoscopic myotomy for treatment of achalasia. Dig Endosc 2016;28:27-32. [Crossref] [PubMed]
- Ujiki MB, Yetasook AK, Zapf M, et al. Peroral endoscopic myotomy: a short-term comparison with the standard laparoscopic approach. Surgery 2013;154:893-7; discussion 897-900. [Crossref] [PubMed]
- Safety of long-term ppi use. JAMA 2017;318:1177-8. [Crossref] [PubMed]
- Falkenback D, Johansson J, Oberg S, et al. Heller's esophagomyotomy with or without a 360 degrees floppy Nissen fundoplication for achalasia. Long-term results from a prospective randomized study. Dis Esophagus 2003;16:284-90. [Crossref] [PubMed]
- Richards WO, Torquati A, Holzman MD, et al. Heller myotomy with Dor fundoplication for achalasia: a prospective randomized double-blind clinical trial. Ann Surg 2004;240:405-12. [Crossref] [PubMed]
- Kummerow Broman K, Phillips SE, Faqih A, et al. Heller myotomy versus heller myotomy with dor fundoplication for achalasia: long term symptomatic follow-up of a prospective randomized controlled trial. Surg Endosc 2018;32:1668-74. [Crossref] [PubMed]
- Rebecchi F, Giaccone C, Farinella E, et al. Randomized controlled trial of laparoscopic heller myotomy plus dor fundoplication versus nissen fundoplication for achalasia: long term results. Ann Surg 2008;248:1023-30. [Crossref] [PubMed]
- Torres-Villalobos G, Coss-Adame E, Furuzawa-Carballeda J, et al. Dor vs toupet fundoplication after laparoscopic heller myotomy: long-term randomized controlled trial evaluated by high-resolution manometry. J Gastrointest Surg 2018;22:13-22. [Crossref] [PubMed]
- Rawlings A, Soper NJ, Oelschlager B, et al. Laparoscopic dor versus toupet fundoplication following heller myotomy for achalasia: results of a multicenter, prospective, randomized-controlled trial. Surg Endosc 2012;26:18-26. [Crossref] [PubMed]
- DeHaan RK, Frelich MJ, Gould JC. Limited hiatal dissection without fundoplication results in comparable symptomatic outcomes to laparoscopic heller myotomy with anterior fundoplication. J Laparoendosc Adv Surg Tech A 2016;26:506-10. [Crossref] [PubMed]
- Simić AP, Radovanovic NS, Skrobic OM, et al. Significance of limited hiatal dissection in surgery for achalasia. J Gastrointest Surg 2010;14:587-93. [Crossref] [PubMed]
- Costantini M, Salvador R, Capovilla G, et al. A thousand and one laparoscopic heller myotomies for esophageal achalasia: a 25 year experience at a single tertiary center. J Gastrointest Surg 2019;23:23-35. [Crossref] [PubMed]
- Rosen MJ, Novitsky YW, Cobb WS, et al. Laparoscopic heller myotomy for achalasia in 101 patients: can successful symptomatic outcomes be predicted? Surg Innov 2007;14:177-83. [Crossref] [PubMed]
- Ross SW, Oommen B, Wormer BA, et al. National outcomes of laparoscopic heller myotomy: operative complications and risk factors for adverse events. Surg Endosc 2015;29:3097-105. [Crossref] [PubMed]
Cite this article as: Kavanagh R, Nau P. Laparoscopic Heller myotomy—A review of the literature. J Xiangya Med 2019;4:12.