
The concept of the special form perforator flap and its role in the evolution of reconstruction
Introduction
Reconstruction of complex soft tissue defects, especially those involving exposure of deep vital structures, remains a challenging problem for reconstructive surgeons (1,2). Several surgery protocols have been described in the previous literature for the reconstruction of complex tissue defects, including local cutaneous flaps, pedicled fasciocutaneous flaps, muscle flaps and free flaps (1-5). However, the use of traditional flap has some disadvantages, including bulking contour and higher donor site morbidity.
Ideal reconstruction surgery should not only repair the defects but also result in limited donor site morbidity and good function recovery (6,7). The perforator flap has enabled to avoid sacrifice of the muscle, the main vessels, deep fascia by using a meticulous dissection and anastomosis (8). Perforator flaps have been performed in increasing numbers since Koshima and Soeda first described perforator flaps in 1989 (9). Multiple clinical series have been reported that perforator flap can be used to realize “like with like” reconstruction of the wound (10-12). However, it is difficult to utilize those methods to reconstruct a complex defects because of their limited soft-tissue amount and less versatile design (13). To overcome the limitations of traditional perforator flap, multiple traditional perforator flaps in combination in a single operation are frequently adopted to repair the complex defects of extremities (14,15). However, more than one microsurgery anastomosis of vascular was required for this procedure, and leading to longer time consume and higher donor site morbidity.
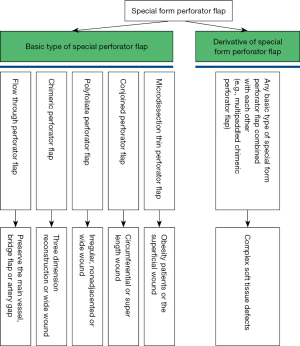
Recently, several special form perforator flaps have been developed for complex soft tissue defect repair (16-19). Those perforator flaps were designed based on the characteristics of the wound and its reconstruction requirements, and then apply minimally invasive techniques and aesthetic concepts of perforator flaps to harvest the bone, skin, fascia or muscle segmentation and enables it to achieve the three-dimensional reconstruction of wound. It also can further improve clinical curative effect and reduce the donor site morbidity through microdissection technique. It is commonly considered that five types of perforators flap including flow-through perforator flap (18), chimeric perforator flap (20), conjoined perforator flap (17), micro-dissected perforator flap (21) and poly-leaf perforator flap (22) as the basic type of the special form perforator flap. Notably, surgeons also can extend the indication with multiple techniques in combination.
The purpose of this paper was to summarize our 10 years experiences on the application of perforator flap. We also introduce a new technique about the special form perforator flap and to explore its application indication.
Methods
Flow-through perforator flap
Severe injury to the extremities usually companied with damage to major vessels (23). It is necessary to restore the damaged vascular system and preserve recipient flow to salvage the damage extremities. In this context, the flow-through technique was one of most popular methods, as it can provide one single operative for arterial reconstruction and soft-tissue coverage simultaneously (24). Moreover, the use of flow-through technique can provide more natural branching of the pedicle through normal physiologic flow and more hemodynamically stable. Thus, flow-through perforator flap provided alternative option for pedicle anastomosis with less flap relation complication and no sacrifice of any more recipient vessels (18).
The flow-through flap was first introduced by Soutar et al. (25) in 1983, and then Koshima and his coworks (24) have described the radial forearm flow-through flap which was used to reconstruct an extremity with a simultaneous vascular defect. Since then, various types of flow-through technique have been introduced (26-29). Most previous literatures have focused on using the flow-through technique for simultaneous vascular gap and soft-tissue defect reconstruction. However, according to our practice clinical experience, the flow-through flaps can be performed for various reconstruction purposes (18). Firstly, flow-through perforator flaps are indicated for revascularization of extremities with concomitant soft-tissue deficits; secondly, flow-through perforator flaps can also be used to preserve the flow integrity of the recipient vessel during free tissue transfer with less recipient site morbidity; thirdly, This technique may be useful for free flap transfer when a suitable recipient vein cannot be found; lastly, flow-through perforator flaps also can be used to provide an arterial source for an additional flap.
Operative technique
This preoperative procedure is similar with the traditional perforator flap. Perforator vascular should be detected in the donor site by using the Hand Doppler probe or CTA as much as possible. To evaluate the vascular anatomy of the recipient site, an extremity CTA was used for all patients. A specific customized reconstruction of individual patients also was performed by our surgery team by assessing and classifying the extremity defects.
Based on the perforators located by Doppler-delineated preoperative and three-dimensional features of the wound, the perforator flap was designed on the donor site. Dissection was performed from the lateral border of the flap, and then a suprafascial dissection until the perforator was identified. Notably, more than one appropriate perforators should been preserved during the dissection process. The perforator was traced back to the main trunk of the donor site vessels, which was dissected according to pedicle length requirements. The large muscle branch should be preserved to perform the T anastomosis (Figure 1).
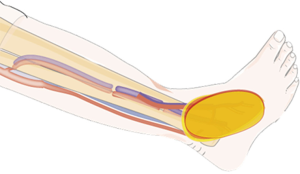
To reconstruct the major artery gap, the pedicle vessels of the perforator flap were inserted into the vascular gap in the extremity. And then micro-anastomosis was performed between the donor artery and the recipient artery. Notably, only the proximal end of the concomitant vein was required to performed micro-anastomosis which will maintain venous drainage of the flap (Figure 2).
To not sacrifice the main recipient vessels and reduce the recipient site morbidity, the flow-through technique was performed to preserve the recipient artery flow. Two ends of the artery pedicle of flow-through flap were anastomosed to two ends of the recipient vessel, while the concomitant vein only needed connect with the proximal of the recipient vein.
For the reconstruction of circumferential soft-tissue defects, the superficial veins of the extremities maybe not healthy. In this situation, preserving the deep veins flow is indispensable to avoid the extremities necrosis. Therefore, the artery and concomitant deep veins of the extremities should be bridged simultaneously to preserve venous return in the leg.
The flow-through perforator flaps can also be performed in combination with another free flap (bridge flap) to cover very large skin defects. The proximal end of the pedicle of flow-through flap was connected with the recipient artery, and the distal end of the pedicle of flow-through flap was connected with the pedicle of an additional flap.
Flow-through perforator flaps can be designed for various purposes in microsurgical reconstruction, including preserving recipient flow, reconstructing an artery gap, repairing an artery and concomitant vein simultaneously, and providing an arterial source for an additional flap. Although various indications may be suitable for flow-through perforator flaps, we considered that the best indication for such a procedure is in patients with single-artery extremities.
Chimeric perforator flap
Complex soft tissue defect reconstruction demands a flap with three-dimensional requirement of both volume and surface (15,30). Traditional perforator flaps may not be large enough or lack the versatility that offer adequate tissue volume and allows precise tissue positioning to optimally cover the wound (19). Reconstruction with three-dimensional microvascular flaps is often the preferred alternative. Chimeric perforator flap can own different tissue component to realize three-dimensional reconstruction.
Chimeric perforator flap consists of multiple independent tissue flaps and each pedicles of flap are linked to a common source vascular. The type of flap was first described as a Siamese combined flap. In 1995, Koshima (23) described it as a mosaic flap which was used for the reconstruction of massive cervical defects, this flap consists of two adjacent free flaps that are simultaneously harvested and whose pedicles were anastomosed as a bridge flap. The nomenclature always is chaos until Huang (31) and Hallock (32) have definite this flap as a chimeric perforator flap in 2003. And then, Hallock further systematic introduced the chimeric concept of compound flaps that are based on different blocks of tissue supplied by different branches of vessels with a common pedicle (16).
Operative technique
This preoperative procedure is similar with the traditional perforator flap. Perforator vascular should be detected in the donor site by using the Hand Doppler probe or CTA as much as possible. To evaluate the vascular anatomy of the recipient site, an extremity CTA was used for all patients. A specific customized reconstruction of individual patients also was performed by our surgery team by assessing and classifying the extremity defects. Enough separately and suitable perforators were detected on at least one thigh. The area of the tissue defect was preoperative estimated, and then a paper template was created according to the feature of the defect to assist the planning of the eventual size of flap.
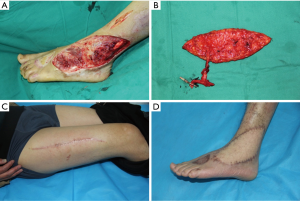
The surgical techniques, which were used to harvest the chimeric perforator flap, were performed as it was described in elsewhere (22,33). Based on the perforators located by Doppler-delineated and three-dimensional features of the wound, the flap was designed on the anterolateral aspect of the thigh. A segment of muscle, bone, nerve or fascia could be included according to trails of defect. For example, a muscle flap can be designed to obliterate the dead space and the cutaneous flap is used to coverage the wound. The chimeric perforator flap can be three-dimensional inset with more degrees of freedom (Figure 3).
Polyfoliate perforator flap
Primary closure of the donor site after flap harvest is the key maneuver in achieving satisfactory results for both patient and operator. The polyfoliate perforator flaps have been recently introduced as a reliable method of extensive soft tissue defects reconstruction with favorable outcomes (34-37). The polyfoliate perforator flap is similar to the concept of chimeric perforator flap which was described by Hallock (20,38). The polyfoliate perforator flap is designed that each skin paddle is supplied by a separate skin perforator, both originating from the same source vessel. The whole flap can be divided into two or more paddles between the perforators, allowing the those paddles to be stacked side by side or rotated about each other, providing cover for larger defects which is impossible to be covered with a convention perforator flap (22). This procedure provided a sufficient area of soft tissue skin for very large defects reconstruction without donor sites skin graft (33). However, until now, the operative technique has not been systematically evaluated.
Preoperative
It is very important to assess the vascular anatomy of donor site for the polyfoliate perforator flap, because More than two suitable perforators, enough long pedicle for each paddle and deriving from the same source vessels are inevitable condition for success elevation a polyfoliate perforator flap. A lower extremity CTA scan was performed for all patients in our department. The localization of perforator was performed as it is described by Chen et al. (39).
Operative technique
After debridement, a paper template with the same dimensions as defect was created. According to the shape of the wound, the laxity of the skin over the anterolateral aspect of the thigh and location of perforators, the separate paddles were designed depend on the various perforators. Dissection was started from the lateral border of the flap, followed by a suprafascial dissection until the perforator was identified. At least two appropriate perforators were selected. The perforator was traced back to the main trunk, and was dissected according to pedicle length requirements. The skin paddles are not split between the perforators until enough and suitable perforators are identified.
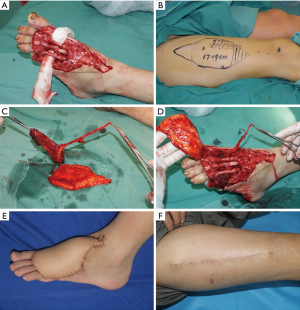
To reconstruct the extensive soft tissue defects, the skin paddles could be placed side by side to extend the width of the flap. This method allows the flap to be used for reconstruction of very large defects whilst maintaining donor-site direct closure. For irregular defect or nonadjacent defects reconstruction, the split paddles can be three-dimensional inset with more degrees of freedom. However, the effective vascular pedicle length between the paddles and the trunk pedicle length should be fully considered to minimize the pedicle tension and twisting (Figure 4).
Conjoined perforator flap
Each perforator holds a unique vascular territory. The vascular territory was named as perforasomes (12,40). Basic on the concept of perforasomes or even angiosomes, Conjoined perforator flaps can allow the creation of incredibly huge flaps that can capture multiple contiguous perforasomes, or even angiosomes. The concept of this type of special form perforator flap was first reported by the Hallock in 2006 (32). They have defined the conjoined perforator flaps as multiple flap territories and dependent because of some common physical junction, yet each retaining its independent vascular supply. More than one of perforasomes or even angiosomes have been included in the Conjoined perforator flap, and a Turbocharge or Supercharge technique is necessary to reestablish the blood supply (41-44). The conjoined perforator flap is more suitable to be used to reconstruct the long but not widen defect. A circumferential soft-tissue defect is another indication for the type of special form perforator flap.
Operative technique
This preoperative procedure is similar with the traditional perforator flap. Perforator vascular should be detected in the donor site by using the Hand Doppler probe or CTA as much as possible.
The surgical technique used to harvest the conjoined perforator flap was similar as the traditional perforator flap (22,33). However, some little tips should be taken careful during the dissection of flap. On the one hand, enough perforator vascular (diameter >0.5 mm) should be hold as much as possible; on the other hand, each perforator should be reliable, and it is inevitable to use vascular clamp to confirmed each perforator territory in order to choice suitable perforator; In additional, when the pedicle was elevated, a large branch of the main vascular should be retained.
To rebuild the blood circulation of conjoined perforator flap, supercharge or turbocharge technique should be adopted during the operation. In the specific situation, more than two perforators were included in a super long conjoined perforator flap, combination supercharge with turbocharge technique should be performed to avoid the partial necrosis in the flap (Figure 5).
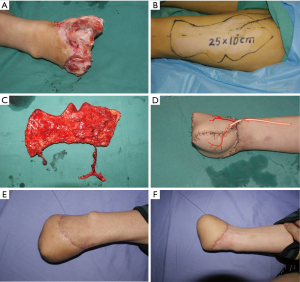
It was special suitable to use the “Turbocharge” flap when only one suitable can be found in the recipient site. “Turbocharge” technique was first described by 1994 by Semple (45) to augment territory of a single pedicle Transverse rectus abdominals myocutaneous flap. Using the T-anastomosis technique, the conjoined perforator flap is able to vascular the skin territory of indirect perforator. The proximal of direct perforator pedicle was anastomosed to the recipient artery, and the distal of direct perforator pedicle was anastomosed to the indirect perforator pedicle. A large muscle branch can also be preserved to bridge the indirect perforator vessels.
When multiple of vessels can be choose in the recipient site, the “surpercharge” flap may be become more suitable. This technique have first introduce by Takayanagi (46) in 1993. The direct perforator pedicle and the indirect perforator pedicle were anastomosed to different recipient vessels respectively.
Microdissection thin perforator flap
Depending on the anatomical location, a thinner flap was more suitable for the soft tissue defect reconstruction in the foot and hand. Numbers of free perforator flap, which have been reported in the recently, can be choice for foot and hand reconstruction. However, Regardless of the available options, these have often resulted in bulk contour and unstable mobile surface. Some author argument that second stage debulking procedure can provide long-term constant, reliable, thin skin coverage for the feet after free flap or local flap reconstruction, but those procedures require another surgery and would not provide a durable over time. The use of Microdissection perforator flap could realize primary thinned-out flaps for reconstruction, it has been shown to result in better contour in hand, foot, head and neck reconstruction (21,47). In 2002, Kimura first introduce a new technique, named “microdissection”, to create a thinner perforator flap by elevating the tensor fasciae latae perforator flap to serve as microdissected thin perforator flap by applying this meticulous technique to the dissection of small vessels in the adipose layer (48). Eleven patients have been included in the article and presented satisfactory outcomes.
Operative technique
This preoperative procedure is similar with the traditional perforator flap. A dominant perforator vascular should be located by using the Hand Doppler probe.
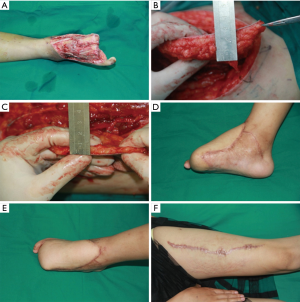
According to characterize of wound, a template was made to assistant outline the flap in the donor site. Along on both sides of the long axis of the flap, dissection was started from the border of the flap, followed by a suprafascial dissection until the perforator was identified. Microscopy or magnifying glass was required for dissection in order to protect the perforator vascular. Basic on the anatomical of source vessels and the design of the flap, dissection of the pedicle beneath the deep fascia is carried out through an extended incision.
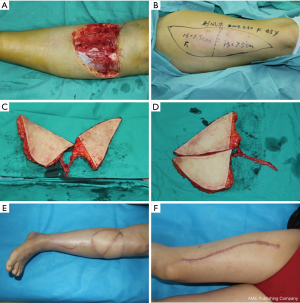
Microdissection into the adipose layer was performed in the deep adipose layer to the level of the superficial adipose layer. Meticulous manipulation was required to protect those branches which were derived from the perforators during the microdissection procedure. Once the fat lobules were removed from around the perforators by the microdissection procedure, we can boldly remove fat tissue according to the needs of regions. Notable, the pedicle should not be cut until the dublking procedure was completed. In additional, circulation of the flap should be checked before cutting the pedicle. This procedure can preserve enough durable thick skin with protective sensation, adequate soft-tissue cushioning, and also get a good anatomical contour, but it is more time-consuming, and success rates is vary (Figure 6).
Discussion
The perforator flap was first introduced by Koshima et al. (49) and has been proven to be reliable in clinical application. The methods have become the workhorse procedure for plastic reconstructive surgery in the past decade. However, for an ideal soft tissue defect reconstruction, the preparation of the perforator flap should be fitted to the details of the wound as accurately as possible. A traditional perforator flap may not be suitable to be obtained for the complex defect reconstruction, therefore, it is necessary to modify the flap not only by elevating the flap precisely to minimize the donor site morbidity but also by adjusting the thickness, bending, folding, and division of the perforator flap. Special form perforator flap can be individual designed with based on the characteristics of the wound and its reconstruction requirements.
Special form perforator flap owns many advantages for the extremities defect reconstruction. On the one hand, according to the characteristic of the defect, special form perforator flap can be designed with free style. For through-and-through defect and multiple nonadjacent defects reconstruction, the split paddles can be three-dimensional inset with more degrees of freedom. To reconstruct the extensive soft tissue defects, the skin paddles can be placed side by side, effectively doubling the width of the flap. We can also achieve soft tissue reconstruction and vascular gap repairing simultaneously with the flow-through perforator flap. On the other hand, special form perforator flaps is ability to simultaneously provide multiple tissue types as building blocks of virtually unlimited size to fill any volume deficit, restore any absent underlying framework, and allow immediate coverage. The ultimate shape and contour can be independently customized and then inset with not limitation, especially with the chimeric perforator flap and polyfoliate perforator flap. In additional, all necessary components of the special fore perforator flap can be obtained from a single donor site that will then be directly closed to minimize donor site morbidity. Meanwhile, if multiple independent parts arise from a single mother major source vessel, a free special form perforator flap will require only a single recipient vessel to revascularize the whole flap. Otherwise, a paucity of recipient vessels will be a significant limiting factor.
Although many advantages of the special form perforator flap have been demonstrated in many papers, there are some tricks and disadvantages which should be taken carefully. First, the vascular anatomy of donor site must be identified before operation. A computed tomography-assisted angiography scan and a handheld Doppler probe were performed in all patients. This is part of our routine preoperative work for special perforator flap transfer. Suitable perforators, the effective vascular pedicle length and each perforator with a same source vascular were necessary to harvest a special form perforator flap. Second, more perforators and meticulously perforator dissecting are requirement during the operation. Hence, longer surgery time is consumed. Thirdly, application of the special form perforator flap requires long learning curve and skillful microsurgery technique, because we generally decide which type of special perforator was used according to the characteristic of the defect and the surgeon’s experience. In additional, more perforator needs to be dissected during the procedure. Lastly, there are also some views that the use of some special form perforator flap has some disadvantages, including the addition of another scar at the recipient site, increasing the risk of kinking or twisting the vascular pedicle and requiring more operation time.
Conclusions
An ideal reconstructive procedure should obtain a satisfactory aesthetic result, better functional recovery, less recipient site complications and limitation of donor site morbidity. Perforator flaps provide a more aesthetically pleasing flap contour and minimizes donor site morbidity. Special form perforator flap is the derivative of the perforators flap, it further raised the clinical curative effect of the perforators flap, and expanded its indications. However, the use of the special form perforator flap requires a long learning curve and skillful microsurgery technique. In the hand of skilled surgeons with extensive microsurgery experience of the traditional perforator flap, special form perforator flaps have been proven to result in less donor-site morbidity, thinner flaps, better patient satisfaction and no difference in recipient-site complications. An algorithm to determine which special perforator flap harvesting for complex extremities soft tissue defect reconstruction should be performed is provided (Figure 7).
Acknowledgments
Funding: This publication was funded in part by the National Natural Science Foundation of China (81472104).
Footnote
Provenance and Peer Review: The article was commissioned by the editorial office, Journal of Xiangya Medicine for the series “Perforator Flap”. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jxym.2019.01.01). The series “Perforator Flap” was commissioned by the editorial office without any funding or sponsorship. JYT served as the unpaid Guest Editor of the series. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by institutional ethics board. Informed consent was waived due to the retrospective nature of the study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Zhu YL, He XQ, Wang Y, et al. Traumatic Forefoot Reconstructions With Free Perforator Flaps. J Foot Ankle Surg 2015;54:1025-30. [Crossref] [PubMed]
- Lee JH, Kang HW, Kim SM, et al. Simultaneous Reconstruction of Forefoot and Hindfoot Defects with a Thoracodorsal-Axis Chimeric Flap. Arch Plast Surg 2015;42:810-3. [Crossref] [PubMed]
- Wang CY, Chai YM, Wen G, et al. Superficial peroneal neurocutaneous flap based on an anterior tibial artery perforator for forefoot reconstruction. Ann Plast Surg 2015;74:703-7. [Crossref] [PubMed]
- Gunnarsson GL, Jackson IT, Westvik TS, et al. The freestyle pedicle perforator flap: a new favorite for the reconstruction of moderate-sized defects of the torso and extremities. Eur J Plast Surg 2015;38:31-6. [Crossref] [PubMed]
- Tajsic N, Winkel R, Husum H. Distally based perforator flaps for reconstruction of post-traumatic defects of the lower leg and foot. A review of the anatomy and clinical outcomes. Injury 2014;45:469-77. [Crossref] [PubMed]
- Fischer S, Klinkenberg M, Behr B, et al. Comparison of donor-site morbidity and satisfaction between anterolateral thigh and parascapular free flaps in the same patient. J Reconstr Microsurg 2013;29:537-44. [Crossref] [PubMed]
- Townley WA, Royston EC, Karmiris N, et al. Critical assessment of the anterolateral thigh flap donor site. J Plast Reconstr Aesthet Surg 2011;64:1621-6. [Crossref] [PubMed]
- Geddes CR, Morris SF, Neligan PC. Perforator flaps: evolution, classification, and applications. Ann Plast Surg 2003;50:90-9. [Crossref] [PubMed]
- Koshima I, Soeda S. Inferior epigastric artery skin flaps without rectus abdominis muscle. Br J Plast Surg 1989;42:645-8. [Crossref] [PubMed]
- Zhang YX, Hayakawa TJ, Levin LS, et al. The Economy in Autologous Tissue Transfer: Part 1. The Kiss Flap Technique. Plast Reconstr Surg 2016;137:1018-30. [Crossref] [PubMed]
- Saint-Cyr M, Wong C, Schaverien MV, et al. The Perforasome Theory: Vascular Anatomy and Clinical Implications. Plast Reconstr Surg 2009;124:1529-44. [Crossref] [PubMed]
- Saint-Cyr M, Schaverien M, Wong C, et al. The extended anterolateral thigh flap: anatomical basis and clinical experience. Plast Reconstr Surg 2009;123:1245-55. [Crossref] [PubMed]
- Brunetti B, Tenna S, Aveta A, et al. Free-style local perforator flaps: versatility of the v-y design to reconstruct soft-tissue defects in the skin cancer population. Plast Reconstr Surg 2013;132:451-60. [Crossref] [PubMed]
- Zelken JA, Chang NJ, Wei FC, et al. The combined ALT-groin flap for the mutilated and degloved hand. Injury 2015;46:1591-6. [Crossref] [PubMed]
- Xu Z, Zhao XP, Yan TL, et al. A 10-year retrospective study of free anterolateral thigh flap application in 872 head and neck tumour cases. Int J Oral Maxillofac Surg 2015;44:1088-94. [Crossref] [PubMed]
- Hallock GG. The complete nomenclature for combined perforator flaps. Plast Reconstr Surg 2011;127:1720-9. [Crossref] [PubMed]
- Hallock GG. Branch-based conjoined perforator flaps. Plast Reconstr Surg 2008;121:1642-9. [Crossref] [PubMed]
- Qing L, Wu P, Liang J, et al. Use of Flow-Through Anterolateral Thigh Perforator Flaps in Reconstruction of Complex Extremity Defects. J Reconstr Microsurg 2015;31:571-8. [Crossref] [PubMed]
- Tang J, Fang T, Song D, et al. Free deep inferior epigastric artery perforator flap for reconstruction of soft-tissue defects in extremities of children. Microsurgery 2013;33:612-9. [Crossref] [PubMed]
- Hallock GG. An introduction to the chimeric deep inferior epigastric artery perforator (DIEAP)-rectus abdominis muscle flap. Ann Plast Surg 2008;61:580-3. [Crossref] [PubMed]
- Dabernig J, Watson S, Hart A. Free microdissected thin groin flap design with an extended vascular pedicle; thin anterolateral thigh perforator flap using a modified microdissection technique. Plast Reconstr Surg 2007;119:2327-8; author reply 2328. [Crossref] [PubMed]
- Marsh DJ, Chana JS. Reconstruction of very large defects: a novel application of the double skin paddle anterolateral thigh flap design provides for primary donor-site closure. J Plast Reconstr Aesthet Surg 2010;63:120-5. [Crossref] [PubMed]
- Koshima I, Fujitsu M, Ushio S, et al. Flow-through anterior thigh flaps with a short pedicle for reconstruction of lower leg and foot defects. Plast Reconstr Surg 2005;115:155-62. [PubMed]
- Koshima I, Saisho H, Kawada S, et al. Flow-through thin latissimus dorsi perforator flap for repair of soft-tissue defects in the legs. Plast Reconstr Surg 1999;103:1483-90. [Crossref] [PubMed]
- Soutar DS, Scheker LR, Tanner NS, et al. The radial forearm flap: a versatile method for intra-oral reconstruction. Br J Plast Surg 1983;36:1-8. [Crossref] [PubMed]
- Sananpanich K, Tu YK, Kraisarin J, et al. Flow-through anterolateral thigh flap for simultaneous soft tissue and long vascular gap reconstruction in extremity injuries: anatomical study and case report. Injury 2008;39:47-54. [Crossref] [PubMed]
- Uraloglu M, Uysal AC, Alagoz MS, et al. Flow-through anterior thigh flaps with a short pedicle. Plast Reconstr Surg 2006;117:2079-80. [Crossref] [PubMed]
- Mureau MA, Flood SJ, Hofer SO. Total peroneal artery occlusion during fibula free flap harvesting: salvage using the venous flow-through principle. Plast Reconstr Surg 2006;117:101e-106e. [Crossref] [PubMed]
- Bullocks J, Naik B, Lee E, et al. Flow-through flaps: a review of current knowledge and a novel classification system. Microsurgery 2006;26:439-49. [Crossref] [PubMed]
- Youn SK, Kim SW, Kim YH, et al. The composite anterolateral thigh flap for achilles tendon and soft tissue defect reconstruction with tendon repair by fascia with double or triple folding technique. Microsurgery 2015;35:615-21. [Crossref] [PubMed]
- Huang WC, Chen HC, Wei FC, et al. Chimeric flap in clinical use. Clin Plast Surg 2003;30:457-67. [Crossref] [PubMed]
- Hallock GG. Further clarification of the nomenclature for compound flaps. Plast Reconstr Surg 2006;117:151e-160e. [Crossref] [PubMed]
- Tsai FC, Yang JY, Mardini S, et al. Free split-cutaneous perforator flaps procured using a three-dimensional harvest technique for the reconstruction of postburn contracture defects. Plast Reconstr Surg 2004;113:185-93; discussion 194-5. [Crossref] [PubMed]
- Zhang YX, Xi W, Lazzeri D, et al. Bipaddle Radial Forearm Flap for Head and Neck Reconstruction. J Craniofac Surg 2015;26:350-3. [Crossref] [PubMed]
- Li KW, Liu J, Liu MJ, et al. Free multilobed posterior interosseous artery perforator flap for multi-finger skin defect reconstruction. J Plast Reconstr Aesthet Surg 2015;68:9-16. [Crossref] [PubMed]
- Zhang YX, Qian Y, Pu Z, et al. Reverse bipaddle posterior interosseous artery perforator flap. Plast Reconstr Surg 2013;131:552e-562e. [Crossref] [PubMed]
- Zhang YX, Messmer C, Pang FK, et al. A novel design of the multilobed latissimus dorsi myocutaneous flap to achieve primary donor-site closure in the reconstruction of large defects. Plast Reconstr Surg 2013;131:752e-758e. [Crossref] [PubMed]
- Hallock GG. The combined parascapular fasciocutaneous and latissimus dorsi muscle conjoined free flap. Plast Reconstr Surg 2008;121:101-7. [Crossref] [PubMed]
- Chen SY, Lin WC, Deng SC, et al. Assessment of the perforators of anterolateral thigh flaps using 64-section multidetector computed tomographic angiography in head and neck cancer reconstruction. Eur J Surg Oncol 2010;36:1004-11. [Crossref] [PubMed]
- Taylor GI, Pan WR. Angiosomes of the leg: anatomic study and clinical implications. Plast Reconstr Surg 1998;102:599-616; discussion 617-8. [Crossref] [PubMed]
- Teven CM, Ooi AS, Chang DW, et al. A Novel Strategy to Supercharge a Deep Inferior Epigastric Artery Perforator Flap after Port-a-Cath Removal. Plast Reconstr Surg Glob Open 2016;4:e1031. [Crossref] [PubMed]
- Numajiri T, Sowa Y, Nishino K, et al. Does a vascular supercharge improve the clinical outcome for free jejunal transfer? Microsurgery 2013;33:169-72. [Crossref] [PubMed]
- Puhaindran ME, Sebastin SJ, Peng YP. Salvage of an ischaemic 'kite flap' by an arterial supercharge: a case report. J Plast Reconstr Aesthet Surg 2007;60:570-2. [Crossref] [PubMed]
- Civelek B, Kargi E, Akoz T, et al. Turbocharge or supercharge? Plast Reconstr Surg 1998;102:1303. [Crossref] [PubMed]
- Semple JL. Retrograde microvascular augmentation (turbocharging) of a single-pedicle TRAM flap through a deep inferior epigastric arterial and venous loop. Plast Reconstr Surg 1994;93:109-17. [Crossref] [PubMed]
- Takayanagi S. Extended transverse rectus abdominis musculocutaneous flap. Plast Reconstr Surg 1993;92:757-8. [Crossref] [PubMed]
- Kimura N, Satoh K, Hosaka Y. Tensor fasciae latae perforator flap. Clin Plast Surg 2003;30:439-46. [Crossref] [PubMed]
- Kimura N. A microdissected thin tensor fasciae latae perforator flap. Plast Reconstr Surg 2002;109:69-77; discussion 78-80. [Crossref] [PubMed]
- Koshima I, Fukuda H, Utunomiya R, et al. The anterolateral thigh flap; variations in its vascular pedicle. Br J Plast Surg 1989;42:260-2. [Crossref] [PubMed]
Cite this article as: Qing L, Wu P, Bing Z, Yu F, Pang X, Ding P, Lei Z, Xiao Y, Fu J, Tang J. The concept of the special form perforator flap and its role in the evolution of reconstruction. J Xiangya Med 2019;4:10.


