Esophagectomy for end-stage achalasia
Introduction
Dr. Ernst Heller reported the first successful surgical treatment of achalasia with esophagocardiomyotomy in 1914 (1). Today, over one century later, the majority of patients with achalasia continue to achieve symptomatic relief from this intervention. Achalasia, however, unfortunately remains a chronic, progressive disease without a cure. The treatment options for achalasia have expanded to include an arsenal of medical, endoscopic, and surgical approaches. From calcium channel blockers and injectable botulinum toxin, to pneumatic balloon dilation, peroral endoscopic and laparoscopic myotomy, the management of patients with achalasia remains complex and challenging. Treatment often requires the expertise from an interdisciplinary team, including primary care physicians, gastroenterologists, nutritionists, anesthesiologists, and surgeons. Likewise, diligent longitudinal follow up of these patients is paramount to monitor for recurrent or persistent symptoms.
Approximately 5% of patients with achalasia will progress to end-stage disease (2). These patients have often failed both non-operative interventions and esophagomyotomy. Surgical treatment options are limited to esophagectomy and esophageal replacement. In this brief review, we present the indications, preoperative considerations, operative techniques, and outcomes of patients undergoing esophagectomy for end-stage achalasia.
Indications
The hallmark features of end-stage achalasia are intractable obstructive symptoms despite prior pneumatic dilation or esophagomyotomy and progression to a dilated (≥6 centimeters), tortuous “sigmoid” megaesophagus. Both are indications to consider esophagectomy; however, surgeons must be aware of several critical diagnostic considerations. Intractable or persistent obstructive symptoms after prior dilation or esophagomyotomy may not represent disease progression, but rather reflux esophagitis and stricture formation, inadequate or healed myotomy, a “tight” fundoplication, hiatal hernia, or pseudoachalasia from an obstructing malignancy. This underscores the importance of reevaluating any patient with presumed end-stage achalasia with a thorough history and physical examination, esophagoscopy, esophageal manometry, and contrast-based functional (e.g., barium swallow) and cross-sectional imaging prior to consideration for esophagectomy (3).
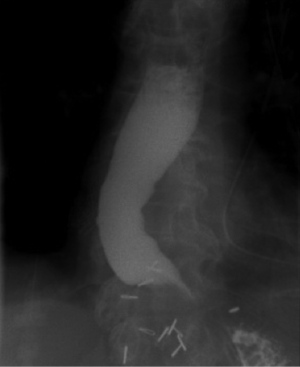
Similarly, in patients who have structurally progressed to megaesophagus, it is important to consider not only size, but also the esophageal orientation. Patients who present with a relatively straight and vertically-oriented megaesophagus (Figure 1) may still benefit from pneumatic dilation or “redo” esophagomyotomy to allow for gravity to assist with esophageal emptying (4). In patients who progress to a tortuous, sigmoid megaesophagus, enlarging or relieving the esophageal outlet is unlikely to improve swallowing or esophageal clearance (Figure 2).
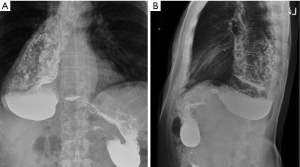
There are several risks associated with chronic retention and stasis of esophageal contents. First, caustic irritation of the esophageal mucosa predisposes to severe esophagitis and formation of peptic strictures. Second, there is a significant risk of concomitant esophageal carcinoma within the megaesophagus (5-7). A recent meta-analysis estimated the prevalence of esophageal carcinoma was 28 cases per 100,000 achalasia patients, correlating with an absolute risk increase of 308 squamous cell carcinoma and 18 adenocarcinoma cases per 100,000 achalasia patients each year (8). Third, patients frequently aspirate from tracheobronchial spillage of retained esophageal contents, causing persistent cough, bacterial and fungal pneumonia, and, in chronic cases, a marked reduction in pulmonary function. Lastly, the lack of esophageal motility coupled with life-altering obstructive symptoms result in nutritional deficiencies and significant weight loss.
In summary, the goals in performing esophagectomy for end-stage achalasia are threefold: (I) to alleviate intractable obstructive symptoms; (II) restore alimentary tract transit and thereby improve nutrition and reduce the risk of aspiration; and (III) remove a defunctionalized megaesophagus where stasis predisposes to esophagitis and malignancy.
Preoperative considerations
Prior to esophagectomy, patients with end-stage achalasia require careful preoperative evaluation and optimization (Figure 3). As stated previously, other potential structural, functional, or malignant etiologies must be excluded through a complete clinical evaluation, esophagoscopy, esophageal manometry, and contrast-based and cross-sectional imaging. These studies will also supplement the surgeon’s operative planning by detailing the altered esophageal and mediastinal anatomy.
Patients with end-stage achalasia are often malnourished as a result of impaired esophageal emptying, inability to tolerate ingested solids or liquids, and the development of food aversion due to intractable symptoms (9). Preoperative coordination with an experienced nutrition team is recommended. Dysphagia diets with nutritional supplements should be encouraged. For patients with severe disease who are unable to tolerate altered diets, a percutaneous or surgical feeding tube may be inserted to initiate non-oral enteric feeding. We recommend a percutaneous or surgical feeding jejunostomy rather than gastrostomy tube, given the need to use the stomach as a potential conduit at the time of esophagectomy.
As a result of chronic aspiration, patients with end-stage achalasia may have compromised pulmonary function. In all cases, pulmonary sepsis should be treated prior to pursuing esophagectomy. Consultation with an experienced team of anesthesiologists familiar with the challenges of induction and single lung ventilation is critical. It is estimated that patients may have 300–500 milliliters of undigested esophageal contents within the dilated megaesophagus (10). As such, massive aspiration can occur during induction of anesthesia or at any point of esophageal manipulation during surgery. Rapid sequence intubation with immediate nasoesophageal decompression by an experienced anesthesiologist is often necessary (11).
Esophagectomy for end-stage achalasia
Surgical approach
Esophagectomy for end-stage achalasia may be completed through a transhiatal or transthoracic approach, and many have reported favorable outcomes for minimally invasive laparoscopic or thoracoscopic techniques (12,13). The choice is ultimately surgeon-dependent, but one must consider the technical challenges of esophagectomy unique to patients with end-stage achalasia. Mobilization of a dilated megaesophagus must occur carefully, under excellent visualization, and with minimal unnecessary manipulation.
The degeneration of the esophageal myenteric plexus and the mass effect of the megaesophagus result in dense scarring and distortion of mediastinal structures. Furthermore, these patients typically have undergone prior botulinum toxin injection, pneumatic dilation, or surgical myotomy. Each contributes to adhesions within the mediastinum and at the esophageal diaphragmatic hiatus. In addition, the hypertrophied esophageal musculature is supplied by a robust network of arterial collaterals, including direct branches from the bronchial arteries and aorta. Poor visualization may therefore lead to significant hemorrhage (14).
These anatomic challenges during esophageal mobilization and resection remain during esophagogastric reconstruction. Both cervical and high intrathoracic esophagogastric anastomoses may be used to restore continuity, though each present unique technical challenges and postoperative complications. While studies have reported a higher rate of stricture formation and recurrent laryngeal nerve injury following cervical esophagogastric reconstruction (15), the potential catastrophic complications from an intrathoracic anastomotic leak, especially in the already-hostile mediastinum of a patient with end-stage achalasia, must be considered.
For these reasons, we advocate for a transthoracic approach to allow for optimal visualization, careful dissection, and hemostasis during esophageal exposure and mobilization. We describe the surgical principles for an open three-incision, or McKeown, esophagectomy using a gastric conduit for esophageal replacement with a cervical esophagogastric anastomosis.
Operative technique
The three-incision esophagectomy occurs in transthoracic, cervical, and abdominal phases (16). An epidural catheter is placed preoperatively. The transthoracic phase begins with double-lumen endotracheal anesthesia, often with rapid sequence intubation and immediate nasogastric tube placement to decompress the megaesophagus and stomach. With the esophagus decompressed, esophagoscopy is performed to further survey for any evidence of esophageal pathology, including malignancy. The patient is then placed in left lateral decubitus position and the right hemithorax is entered through a standard posterolateral thoracotomy over the sixth interspace with single lung ventilation. The megaesophagus is exposed by incising the mediastinal pleura just caudal to the arch of the azygous vein. Dissection toward the diaphragmatic hiatus is accomplished with careful attention to hemostasis given the rich vascular supply to the megaesophagus. We do not routinely ligate the thoracic duct prophylactically.
The dissection is carried superiorly into the thoracic inlet. The azygos vein is routinely divided with a vascular staple load. After complete esophageal mobilization, the right chest is closed after insertion of a thoracostomy tube and direct visualization of right lung re-expansion.
The patient is repositioned in the supine position. It is important to note that aspiration may occur during any repositioning and, therefore, this should be limited to few careful movements. The double-lumen endotracheal tube is replaced with a single-lumen endotracheal tube, and the head is positioned toward the right, exposing the left neck. An incision anterior to the left sternocleidomastoid is made to begin the exposure of the cervical esophagus. Dissection is carried out between the internal carotid artery laterally and thyroid gland medially. The middle thyroid vessels and omohyoid muscle are divided to allow for further medial retraction of the thyroid gland and exposure of the underlying tracheoesophageal groove. While protecting the recurrent laryngeal nerve, the cervical esophagus is encircled, and a combination of sharp and blunt dissection is used to mobilize the cervical esophagus to the level of the apical pleura, marking the superior extent of the thoracic esophageal mobilization.
After completing the cervical esophageal dissection, the abdomen is entered through an upper midline laparotomy and the stomach is mobilized taking care to maintain the right gastroepiploic artery, the vascular pedicle for the gastric conduit. To gain sufficient length for the gastric conduit to reach the left neck, an extensive Kocher maneuver is performed to further mobilize the duodenum. Potentially complicating the abdominal phase of the operation is any prior fundoplication performed during previous myotomy. In these cases, a portion of the fundus has been used in an anterior-posterior fundoplication, requiring careful dissection of the stomach to preserve maximal length. With the stomach completely mobilized, the gastroesophageal junction is divided with a stapler, allowing the esophagus to be removed via the cervical incision. A neo-esophagus is fashioned from the stomach by using a linear stapler along the greater curvature, creating a conduit 5–6 cm in width. We do not presently use a gastric emptying procedure, but would perform a pyloromyotomy at this point per surgeon preference.
The gastric conduit is then pulled toward the left neck by passing a chest tube from the cervical incision into the abdomen through the esophageal hiatus, followed by a laparoscopic camera bag to atraumatically draw the gastric conduit upward into the mediastinum. It is important to emphasize that the conduit is maintained in proper orientation without twisting or torsion of any fashion. Then, an end-to-side cervical esophagogastric anastomosis is fashioned using a circular stapler with the anvil secured in the cervical esophagus with several purse-string sutures, followed by the handle placed through the tip of the gastric conduit. A side-to-side stapled cervical esophagogastric anastomosis may also be performed with adequate length of the gastric conduit (17). With the cervical anastomosis completed, the neck is closed with several interrupted sutures. If not present preoperatively, a jejunostomy tube may be inserted prior to abdominal closure.
In cases of prior gastric resection or when the stomach cannot be used as a conduit, alternative intestinal conduits may be created. Colonic conduits provide adequate length, resist to environments, and are associated with fair to good functional outcomes despite impaired peristalsis and lack of absorptive capacity (4,18). Jejunal conduits maintain peristalsis and a degree of absorptive capacity; however, the vascular supply, even when “supercharged”, potentially limit the length of jejunal interposition (19). Both require multiple intestinal anastomoses. We recommend the stomach should be used when possible given its rich blood supply, requirement of only a single esophagogastric anastomosis, and superior functional outcomes.
Postoperative care and complications
Patients are extubated intraoperatively and monitored in an intensive care unit for the first postoperative day. Tube feeds are initiated and slowly advanced on the second postoperative day. The nasogastric tube remains in place until the fifth postoperative day, at which time a contrast-based swallow study is obtained. If there is no evidence of an anastomotic leak, the nasogastric tube is removed and the patient is initiated on a liquid diet prior to discharge.
Immediate postoperative complications are related to technical challenges during the transthoracic, cervical, and abdominal phases of the three-incision esophagectomy (20). As with any thoracic surgery, early postoperative pneumothorax, chylothorax, aspiration, or cardiac arrhythmia may occur. Patients with end-stage achalasia who have undergone esophagectomy are at greater risk of postoperative hemorrhage given the extensive network of arterial collaterals surrounding the esophagus. Thoracostomy tube output and patency, as well as chest radiographs must be closely monitored for ongoing blood loss. Cervical esophagogastrostomy anastomotic leaks can occur, typically manifesting as systemic response to infection or change in the quantity and quality of cervical drainage. The majority of cervical esophagogastrostomy anastomotic leaks heal with conservative management of nil per os, antibiotics, and continued monitored drainage (21).
Outcomes
Outcomes of esophagectomy for end-stage achalasia are generally favorable. The largest series of esophagectomy for end-stage achalasia report 2–5% overall mortality and approximately 30% morbidity (22). Post-operative complications were related primarily to cervical esophagogastrostomy anastomotic leak. At follow up ranging from 3 to 6 years, 88% of patients were overall satisfied, 91% reported good or excellent outcomes, and 96% tolerated an unaltered diet.
Conclusions
Esophagectomy with esophageal replacement remains the surgical treatment of choice for patients with end-stage achalasia who have failed conservative measures. These patients must be carefully evaluated preoperatively by a multidisciplinary team for accurate diagnosis, pulmonary function, and nutritional optimization. We recommend three-incision esophagectomy with gastric conduit esophageal replacement and cervical esophagogastrostomy given improved exposure and attention to complex mediastinal anatomy, dense periesophageal scarring, and increased vascular collaterals. In general, esophagectomy is successful in improving patient symptoms and can be completed with minimal morbidity and mortality.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (David W. Rattner, Ozanan Meireles) for the series “Update on the Diagnosis and Treatment of Achalasia” published in Journal of Xiangya Medicine. The article has undergone external peer review.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jxym.2019.01.04). The series “Update on the Diagnosis and Treatment of Achalasia” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Haubrich WS. Heller of the Heller Myotomy. Gastroenterology 2006;130:333. [Crossref]
- Molena D, Mungo B, Stem M, et al. Outcomes of Esophagectomy for Esophageal Achalasia in the United States. J Gastrointest Surg 2014;18:310-7. [Crossref] [PubMed]
- Devaney EJ, Iannettoni MD, Orringer MB, et al. Esophagectomy for achalasia: Patient selection and clinical experience. Ann Thorac Surg 2001;72:854-8. [Crossref] [PubMed]
- Mormando J, Barbetta A, Molena D. Esophagectomy for benign disease. J Thorac Dis 2018;10:2026-33. [Crossref] [PubMed]
- Brücher BL, Stein HJ, Bartels H, et al. Achalasia and Esophageal Cancer: Incidence, Prevalence, and Prognosis. World J Surg 2001;25:745-9. [Crossref] [PubMed]
- Loviscek LF, Cenoz MC, Badaloni AE, et al. Early cancer in achalasia. Dis Esophagus 1998;11:239-47. [Crossref] [PubMed]
- Crema E, Ribeiro LB, Terra JA, et al. Laparoscopic Transhiatal Subtotal Esophagectomy for the Treatment of Advanced Megaesophagus. Ann Thorac Surg 2005;80:1196-201. [Crossref] [PubMed]
- Tustumi F, Bernardo WM, da Rocha JRM, et al. Esophageal achalasia: a risk factor for carcinoma. A systematic review and meta-analysis. Dis Esophagus 2017;30:1-8. [Crossref] [PubMed]
- Dughera L, Chiaverina M, Cacciotella L, et al. Management of achalasia. Clin Exp Gastroenterol 2011;4:33-41. [Crossref] [PubMed]
- Calu V, Dutu M. How Would You Manage the Airway in This Case? In: Rosenblatt WH, Popescu WM. Master Techniques in Upper and Lower Airway Management. 1st edition. Philadelphi: Lippincott Williams & Williams, 2015.
- Panda N, Donahue DM. Acute airway management. Ann Cardiothorac Surg 2018;7:266-72. [Crossref] [PubMed]
- Schuchert MJ, Luketich JD, Landreneau RJ, et al. Minimally invasive surgical treatment of sigmoidal esophagus in achalasia. J Gastrointest Surg 2009;13:1026-9. [Crossref] [PubMed]
- F Fontan AJA. Minimally Invasive Laparoscopic Esophagectomy vs. Transhiatal Open Esophagectomy in Achalasia: A Randomized Study. Arq Bras Cir Dig 2018;31:e1382. [PubMed]
- Miller DL, Allen MS, Trastek VF, et al. Esophageal resection for recurrent achalasia. Ann Thorac Surg 1995;60:922-6. [Crossref] [PubMed]
- van Workum F, van der Maas J, van den Wildenberg FJH, et al. Improved Functional Results After Minimally Invasive Esophagectomy: Intrathoracic Versus Cervical Anastomosis. Ann Thorac Surg 2017;103:267-73. [Crossref] [PubMed]
- Heitmiller RF, Skaryak LA. Transthoracic Approach for Achalasia. In: Luketich JD. Master Techniques in Surgery. Philadelphia: Wolters Kluwer Health, 2014:173-82.
- Batista Neto J, Morais PG, Nepomuceno Mda C, et al. Mechanical cervical esophagogastric laterolateral anastomosis after esophagectomies. Rev Col Bras Cir 2013;40:420-2. [Crossref] [PubMed]
- Hsu HS, Wang CY, Hsieh CC, et al. Short-segment colon interposition for end-stage achalasia. Ann Thorac Surg 2003;76:1706-10. [Crossref] [PubMed]
- Bakshi A, Sugarbaker DJ, Burt BM. Alternative conduits for esophageal replacement. Ann Cardiothorac Surg 2017;6:137-43. [Crossref] [PubMed]
- Moores DWO, Rassias DJ. Master Techniques in Surgery: Esophageal Surgery. Luketich JD. editor. Wolters Kluwer Health, 2014.
- Turkyilmaz A, Eroglu A, Aydin Y, et al. The management of esophagogastric anastomotic leak after esophagectomy for esophageal carcinoma. Dis Esophagus 2009;22:119-26. [Crossref] [PubMed]
- Molena D, Yang SC. Surgical Management of End-Stage Achalasia. Semin Thorac Cardiovasc Surg 2012;24:19-26. [Crossref] [PubMed]
Cite this article as: Panda N, Morse CR. Esophagectomy for end-stage achalasia. J Xiangya Med 2019;4:7.