
Diagnostic and therapeutic challenges of sustained ventricular tachycardia revealing cardiac lipoma in sub-Saharan Africa: a case report of a 31-year old woman in Cameroon
Introduction
Sudden death is not infrequent in low-middle income (LMIC) settings (1). Many cases occur in the communities, and autopsies are rarely performed. Most cases of sudden death are cardiovascular in origin (2). Ventricular fibrillation is the most likely cause, either spontaneously or after a sustained ventricular tachycardia (VT) (2). The burden of VT in LMIC settings is not known, and the diagnosis and treatment of these potentially fatal arrhythmias are challenging in these settings. The outcome of VT remains poor, especially in lower level hospitals (1). We report the case of a 31-year-old woman with VT revealing cardiac lipoma seen in a tertiary hospital in sub-Saharan Africa (SSA). The initial presentation was suggestive of peri-partum cardiomyopathy. This rare case highlights the challenges in the diagnosis and management of VT associated with cardiac lipoma in SSA. We report this case according to the standards for reporting case reports (CARE) guidelines.
Case presentation
Mrs B.A.L.P, a 31-year-old mother of four children, who resides in Cameroon. Her past medical history was remarkable for grade 2 dyspnea two years ago, in the early post-partum of her fourth child. Cardiac ultrasound was remarkable for a dilated left atrium, with a mildly reduced left ventricular (LV) ejection fraction (Teicholz method) of 50% (according to the American Society of Echocardiography recommendations). ECG showed sinus rhythm with rare premature ventricular beats (PVCs), PR interval of 240 ms, and QTc of 500 ms. She was lost to follow-up, and was taking no medicines except contraception-subcutaneous progesterone implants, since one year. She experienced occasional shortness of breath with fatigue, yet did not seek medical care. Two weeks prior to admission in our unit, she had a loss of consciousness, associated with agitation and loss of urine. Still she sought no medical care.
She was referred for the management of tachycardia (heart rate of 200 beats per minute) and grade 4 dyspnea, evolving since five hours (September 28, 2013). This was preceded by a sudden onset of palpitation, vertigo, and intense fatigue. Physical examination was remarkable for a blood pressure of 84/47 mmHg, heart rate of 208 beats per minute, and a respiratory rate of 20 cycles per minute. Oxygen saturation was 97% on ambient air, and random blood glucose was 2.3 g/L. She was restless but conscious, with pale and cold extremities. Heart auscultation was unremarkable, and there were no signs of central and peripheral congestion. The rest of the clinical examination was unremarkable.
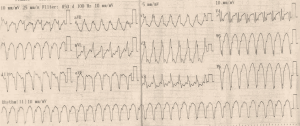
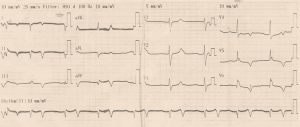
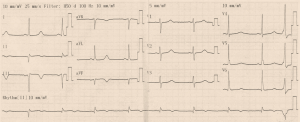
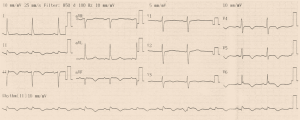
Initial ECG (Figure 1) showed wide QRS complex tachycardia (monomorphic) at 208 cycles per minute, complete right bundle branch block aspect, no fusion nor capture beats, and possible A-V dissociation (P-waves in D1, D3 and aVF) suggestive of ventricular tachycardia (VT). No defibrillator nor IV drug for treating VT was immediately available. We attempted vagal maneuvers and administered 80mg of propranolol orally as the only medicine available. No early improvement was noted. The VT changed to atrial fibrillation six hours later, with inverted T-waves in V4-V6, QTc of 560 ms (Figure 2). Cardiac enzymes showed evidence of myocardial injury: Troponin I: 5.3 ng/mL (<0.2), GOT: 446 UI/L (<31 UI/L), CPK: 553.7 UI/L (27–300 UI/L), CPK-MB: 231.8 UI/L (<25 UI/L), LDH: 627.78 UI/L (200–400 UI/L). CRP was 11.1 mg/L (<6), and hemogramme was unremarkable. She had mild hypokalemia (3.37 mEq/L) and hypocalcemia (86.8 mg/L). Lipid profile and thyroid hormones were normal. Twelve hours later, she was in sinus rhythm, with polymorphic premature ventricular contractions (PVCs), q waves in D2, aVF, and D3, T wave inversion in the lateral leads (Figures 3,4). Cardiac ultrasound showed a reduced ejection fraction (LV Ejection Fraction: 39% Teicholz method), dilated LV (LV end-diastolic diameter: 64 mm) with normal wall thickness, and bi-atrial dilation (Figure 5). Pulmonary pressure was normal (RV-RA gradient: 18 mmHg). She was started on Amiodarone 200 mg daily. Beta-blockers and ACE inhibitors were deferred due to persistent low blood pressure (BP). Holter ECG on amiodarone showed: 1,932 premature ventricular contractions (PVCs), 14 salves of PVCs, 34 doublets of PVCs, 3,823 premature atrial contractions (PACs), and 29 salves of PACs. Her average 24-hour heart rate was 64 cycles per minute, and ranged from 31 to 112. We referred her abroad (Paris-France) for ablation of VT or insertion of a defibrillator.
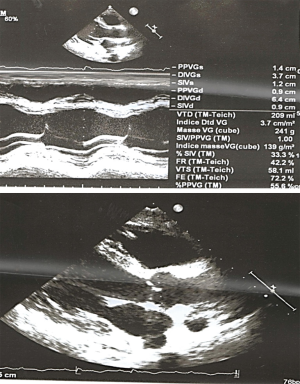
In a tertiary health institution in Paris-France, electrophysiologic studies showed two inducible VT foci in the LV, of which one was successfully ablated. Cardiac MRI showed a cardiac tumor, and surgical excision specimen was suggestive of lipoma. This was confirmed on histopathology analysis. Pre-operative coronarography was normal. She was maintained on amiodarone 200 mg daily, and initiated on bisoprolol 10 mg daily, and ramipril 1.25 mg daily. Her LV ejection fraction improved to 50% before discharge.
Back to Yaounde-Cameroon, she was seen on 22-10-2015 (ten months after her return from abroad). She complained of grade 2 dyspnea, occasional palpitations, and nonspecific chest pain. Her BP was 105/55 mmHg, heart rate of 41 beats per minute, and respiratory rate of 16 cycles per minute. Then, she was lost to follow-up.
Discussion
We present the case of sustained ventricular tachycardia (VT) in a young woman with diagnostic and therapeutic challenges. The echocardiographic presentation was LV dilation. Cardiac MRI suggested a LV free wall tumor. The pathologic diagnosis was LV free wall lipoma.
Few cases of sustained VT have been documented in our setting (1), as most patients die before reaching the hospital. To the best of our knowledge, no case of cardiac lipoma has been reported in patients from our setting. Many cases of sudden death have been noted in low income settings, but the precise cause of death is not known. Few post mortem examinations are carried out routinely. Thus, the precise burden of VT and cardiac lipoma in our setting is not known. Many cases of sudden deaths have been shown to be of cardiovascular origin (2). ECGs to make the diagnosis of VT are not widely available. This case had an ECG performed that was diagnostic of VT. The initial management was very challenging as there was no electrical cardioverter. When available, most HCPs don’t master their use. Intravenous anti-arrhythmic drugs such as IV Amiodarone is not widely available. The availability of IV Lidocaine is not guaranteed, and most formulations are for use as local anesthetics. The manipulation of these medicines in our setting is also challenging, from lack of experience to lack of electric syringes for the gradual administration of medicines. Our initial treatment strategy of vagal maneuvers and 80 mg of oral propranolol was inefficient. The second challenge was the cause or trigger of VT in this young woman. The only diagnostic tool available was cardiac ultrasound, which showed a markedly dilated LV and atria. Cardiac MRI was not available at that time, and the cost is very prohibitive. Since the first episode of heart disease occurred just after delivery, the case was suggestive of peri-partum cardiomyopathy that did not recover. Another big challenge was her long-term management. Long term use of amiodarone in this young patient could result in serious adverse outcomes such as irreversible fibrosis of the lungs, and reversible thyroid dysfunction (3,4). Amiodarone and sotalol are also contra-indicated with long QTc, as they can trigger a polymorphic VT or Torsade de point (5,6). Insertion of a defibrillator and ablation of the VT focus was not available, and remains costly. We deferred the use of beta-blocker and ACE inhibitor due to concerns with blood pressure. Surgical management of this case was also challenging, as this was also not available. In fact, managing VT in a low income setting is associated with an unfavorable outcome, especially in lower level hospitals. Nkoke et al. documented a case of sustained VT with a fatal outcome (1). Oral Amiodarone was the only available strategy. As anecdotes, we had a case of wide QRS complex tachycardia—probably a VT, in a second level hospital with death of the patient 30 minutes after arrival. We managed a case of cardio-circulatory collapse and tachycardia (heart rate with auscultation ≈200 beats per minute) in the emergency department of a third level hospital. No ECG was available for a formal diagnosis of VT. We gave repeated 1 cc doses of diluted Lidocaine (1cc of lidocaine 2% plus 9 cc of normal saline), with resultant slowing of the heart rate (130 to 140 beats per minute), and measured systolic blood pressure of 80 to 100 mmHg. This permitted referral to a tertiary hospital for further care. Another challenge is patient compliance, despite the severity of the condition. The cost of treatment is also another challenge, as patients have to pay out-of-pocket for their care.
The differential diagnosis of large QRS tachycardia is VT, which should be considered first and managed accordingly. A second diagnosis to consider is supraventricular tachycardia with aberrant conduction. Finally, a supraventricular tachycardia on an existing bundle branch block (2). In the absence of a structural heart disease, two benign forms of VT could be considered—the Belhassen type or infra-Hissien VT (7), and Gallavardin type or right infundibular VT (8). The treatment of choice of VT is electrical cardioversion when there is hemodynamic instability (2). Chemical cardioversion can be achieved with IV Amiodarone and other IV anti-arrhythmics (9,10). IV Amiodarone is difficult to manipulate as it is advisable to administer via a central line, due to concerns of tissue necrosis with extravasation. IV Lidocaine is an interesting option as it is more available as local anesthetics in LMIC settings. IV lidocaine has been shown to be as efficient as IV amiodarone (11). But there are no local guidelines for its use.
Cardiac lipoma is a very rare primary cardiac tumor, especially lipoma in the LV. A PubMed search by Sun et al. revealed a total of 20 cases of LV cardiac lipoma (12). To the best of our knowledge, this is the first documented case of cardiac lipoma in our setting. The clinical presentation is nonspecific, often with cardiac (palpitations, shortness of breath, and chest pain) and neurologic manifestations (dizziness and syncope). Some can be totally asymptomatic. This results in misdiagnosis or late diagnosis as reported by Sun et al. (12). Our case presented with cardiac and manifestations, and was misdiagnosed as a peripartum cardiomyopathy with cardiac ultrasound. The diagnosis of cardiac lipoma on cardiac ultrasound can be challenging, especially the invasive or infiltrative forms. This can be well characterized with fat subtraction cardiac MRI (13). The ideal management of an intra cardiac LV tumor is surgical excision.
Conclusions
Ventricular tachycardia revealing cardiac lipoma poses significant diagnostic and therapeutic challenges in low-income settings, with resultant poor outcome especially in lower level hospitals. There is a need to equip hospitals with electrocardiographs and defibrillators, and empower Health Care Professionals on their use. IV anti-arrhythmic medicines should be made available, and local protocols made for their use in case of VT. MRI for proper myocardial tissue study should be made available and accessible at least in tertiary health institutions in low income settings. Facilities for cardiac surgery should be developed.
Acknowledgements
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jxym.2018.11.03). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Nkoke C, Luchuo EB, Dikoume L. Fatal monomorphic ventricular tachycardia in a semi-urban setting in Cameroon: a case report. BMC Res Notes 2017;10:180. [Crossref] [PubMed]
- John RM, Tedrow UB, Koplan BA, et al. Ventricular arrhythmias and sudden cardiac death. Lancet Lond Engl 2012;380:1520-9. [Crossref] [PubMed]
- Anastasiou-Nana MI, Anderson JL, Nanas JN, et al. High incidence of clinical and subclinical toxicity associated with amiodarone treatment of refractory tachyarrhythmias. Can J Cardiol 1986;2:138-45. [PubMed]
- Lee W, Ryu DR, Han SS, et al. Very early onset of amiodarone-induced pulmonary toxicity. Korean Circ J 2013;43:699-701. [Crossref] [PubMed]
- Tong KL, Lau YS, Teo WS. A case series of drug-induced long QT syndrome and Torsade de Pointes. Singapore Med J 2001;42:566-70. [PubMed]
- Roden DM. Drug-induced prolongation of the QT interval. N Engl J Med 2004;350:1013-22. [Crossref] [PubMed]
- Topilski I, Glick A, Belhassen B. Idiopathic left ventricular tachycardia with a right bundle branch block morphology and left axis deviation (“Belhassen type”): results of radiofrequency ablation in 18 patients. Isr Med Assoc J 2004;6:195-200. [PubMed]
- Hoffmann E, Reithmann C, Neuser H, et al. Repetitive monomorphic ventricular tachycardia (Gallavardin type): clinical and electrophysiological characteristics in 20 patients. Z Kardiol 1998;87:353-63. [Crossref] [PubMed]
- Tomlinson DR, Cherian P, Betts TR, et al. Intravenous amiodarone for the pharmacological termination of haemodynamically-tolerated sustained ventricular tachycardia: is bolus dose amiodarone an appropriate first-line treatment? Emerg Med J 2008;25:15-8. [Crossref] [PubMed]
- Goldschlager N, Epstein AE, Naccarelli GV, et al. A practical guide for clinicians who treat patients with amiodarone: 2007. Heart Rhythm 2007;4:1250-9. [Crossref] [PubMed]
- Kudenchuk PJ, Brown SP, Daya M, et al. Amiodarone, Lidocaine, or Placebo in Out-of-Hospital Cardiac Arrest. N Engl J Med 2016;374:1711-22. [Crossref] [PubMed]
- Sun X, Liu G, Kim H, et al. Left ventricular lipoma resected using thoracoscope-assisted limited sternotomy: A case report and literature review. Medicine (Baltimore) 2018;97:e11436. [Crossref] [PubMed]
- D'Souza J, Shah R, Abbass A, et al. Invasive Cardiac Lipoma: a case report and review of literature. BMC Cardiovasc Disord 2017;17:28. [Crossref] [PubMed]
Cite this article as: Jingi AM, Boombhi J, Mfeukeu-Kuate L, Hamadou B, Abas A, Mintom P, Menanga A. Diagnostic and therapeutic challenges of sustained ventricular tachycardia revealing cardiac lipoma in sub-Saharan Africa: a case report of a 31-year old woman in Cameroon. J Xiangya Med 2018;3:41.


