
Successful percutaneous treatment of late-onset femoral pseudoaneurysm after transcatheter aortic valve implantation procedure
Introduction
The vast majority of transcatheter aortic valve implantation (TAVI) procedures are presently done via the transfemoral route. Because of the quite large profile of the delivery system and associated atherosclerotic changes in access vessels, vascular complications, which may lead to life-threatening bleeding and increased morbidity and mortality, remain the main limitation of TAVI (1). A percutaneous vascular closure device is commonly used to achieve the hemostasis of the femoral artery access site. Although failure in percutaneous closure of the femoral access site is expected in the early period, largely due to technical reasons, it is not expected in the late period. In this report, we want share our experience of successful percutaneous treatment of late-onset femoral pseudoaneurysm after TAVI procedure.
Case presentation
A 69-year-old female was admitted to our department with shortness of breath and angina pectoris for the last few months. In the past medical history, she had persistent atrial fibrillation, congestive heart failure, chronic obstructive pulmonary disease, diabetes mellitus and rheumatoid arthritis. Two-dimensional echocardiography revealed a severe aortic stenosis, PG max/mean: 68/48 mmHg, AVA: 0.5 cm2, and moderate aortic regurgitation with systolic ejection fraction of 35%. The functional capacity of patients was NYHA III. After discussion with the surgical team, a surgical option was considered too risky (Euroscore II was 9.02%), and the patient was referred to TAVI. The transfemoral access was chosen due to suitable iliofemoral anatomy and its less invasive nature. After placing the 18 F sheath into the right femoral artery, a 26 mm balloon-expandable Edwards Sapien XT valve (Edwards Lifesciences, Inc., Irvine, CA, USA) was successfully implanted through this way. At the end of the procedure, femoral access site successfully closed with 2 ProGlides (Abbott Vascular Devices, Redwood City, CA, USA).
Mostly due to the co-morbidities, especially chronic obstructive pulmonary disease, the patient was followed up clinically for approximately 12 days. However, hypoxemic attacks despite oxygen replacement were observed and she was decided to transfer to the pulmonary disease clinic according to the consultation result. While followed at pulmonary disease clinic, the patient was consulted to us because of developing a palpable, painful, and pulsatile mass in her right groin (21 days after the TAVI procedure). An ultrasound scan of the right groin showed a 38 mm × 33 mm pseudoaneurysm of the right common femoral artery. After discussion with the surgical team, a surgical option was deemed too risky due patient’s co-morbidities and unexpected rupture risk of pseudoaneurysm. Percutaneous endovascular graft stent placement was decided.
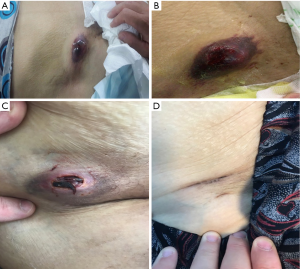
Selective right femoral angiography through the contralateral left femoral puncture confirmed the pseudoaneurysm of the common femoral artery measuring about 5 mm in diameter (Figures 1,2). A 8 mm × 38 mm balloon-expanding Atrium ADVANTA V12 Balloon Expandable Covered Stent (Atrium Medical Corporation, NH, USA) was advanced over the guidewire, by the cross-over technique, through a previously placed cross-over 7 Fr 35 mm vascular sheath and successfully implanted into the targeted place and the pseudoaneurysm disappeared (Figures 1B,3). The patient was transferred to coronary intensive care unit for clinical follow-up. However, it was observed that the palpable, pulsatile mass in her right groin was still palpable in the physical examination made at the next morning. Repeated right femoral angiography via left common femoral artery was performed and revealed an extravasation despite the covered stent in common femoral artery (Figure 1C). When we examined the angiography at different angles in more detail, we observed a folding deformation in the distal segment of the covered stent (Figures 1D,4). Prolonged balloon inflation was done in the previously implanted stent with crossover technique through a 7 Fr long 35 mm vascular sheath from the contralateral left femoral puncture. Final angiography was revealed that right femoral pseudoaneurysm was totally disappeared (Figures 1E,5). The patient was discharged 7 days later with partial improvement. At the second month of follow-up after discharge, complete recovery of the lesion was observed (Figure 6A,B,C,D).
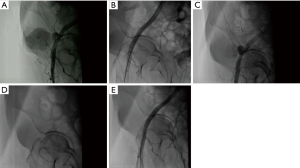




Discussion
There are numerous commonly incontestable anatomic, patient-related and operator-dependent factors, which increase the vascular complication risk. These include advanced age, female gender, high surgical risk score, calcification, increase in body mass index, insulin-treated diabetes mellitus, anticoagulation, combined arterial and venous puncture, failure to provide proper press, smaller vessel diameter, higher sheath-to-femoral artery ratio (sheath-femoral artery ratio >1.05) and sheath-to-external-iliac-artery ratio (6,7). The size of delivery sheath is of course one of the major risk factors of vascular access site complications. Although the larger sheath sizes of first generation delivery systems, the newest ones have smaller sheath sizes. Edward SAPIEN 3 valve and Medtronic CoreValve Evolute require 14-French sheaths. Thereby, the frequency of vascular complications is gradually reducing.
Vascular complication rates after TAVI were predominantly determined by femoral artery injuries, while iliac and aortic injuries were enormously infrequent.
This is especially due to the failure of the vascular closure devices. Percutaneous vascular closure devices presently employed for the hemostasis of femoral access site are the Prostar XL (percutaneous vascular surgical system) and Perclose ProGlide (suture-mediated closure system). Although these vascular closure devices are used with great success in daily clinical practice, failures in percutaneous closure of the femoral access site can be seen rarely, especially when large diameter devices are used, and when it occurs, it is associated with a substantial increase in the risk of vascular complications. In the study of Barbash et al., the composite primary end point of major vascular complications or in-hospital mortality occurred more frequently in patients treated by Prostar when compared with ProGlide (8).
In our case, an 18-French sheath was used. The femoral access site was percutaneously successfully closed with 2 ProGlides and early hemostasis was achieved. However, we observed a late-onset femoral pseudoaneurysm that developed 21 days later at the access site. When we retrospectively analyzed all the procedure and clinical follow-up period, we have not found any technical cause of failure. Nevermore, we observed that arterial blood gas sampling was taken from right femoral artery twice while hospitalizing in pulmonary disease clinic. It may be one of the possible causes of femoral pseudoaneurysm. When we reviewed the literature, we could not find a case report that reported late-onset failure of a vascular closure device. From this point of view, our case is unique one.
Surgery is the traditional treatment method of vascular complication. Although surgical success rate is very high, delayed wound healing and infection, and significant morbidity and mortality rates are the major concerns (9). Percutaneous endovascular treatment of femoral pseudoaneurysm is highly effective and safe with its less invasive nature, especially in high-risk patients. As experience increases, most of the vascular complications associated with the transfemoral approach can be treated percutaneously. In our case, we preferred a balloon-expandable stent graft because of its higher radial strength, and the capability for post-dilation and upsizing after implantation. However, an extravasation due to a folding deformation in the distal segment of the covered stent was observed at the control made at the next day. Afterwards, we thought that a self-expandable graft stent could be more appropriate due to its optimal conformance to underlying vessels in a curved and mobile groin region, and a possibly a better seal could be achieved.
In conclusion, it is reasonable not to use of the percutaneously closed vessel to cause a femoral complications for a period of time. With the percutaneous endovascular treatment appears to be an appropriate option for femoral pseudoaneurysm, it should be well evaluated which graft stent type is suitable, self-expandable or balloon-expandable.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jxym.2018.09.02). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Mangla A, Gupta S. Vascular complications post-transcatheter aortic valve procedures. Indian Heart J 2016;68:724-31. [Crossref] [PubMed]
- Celik M, Yuksel UC. Femoral angiography. Asvide 2018;5:758. Available online: http://www.asvide.com/article/view/27318
- Celik M, Yuksel UC. Stent deployment. Asvide 2018;5:759. Available online: http://www.asvide.com/article/view/27319
- Celik M, Yuksel UC. Stent distal fracture. Asvide 2018;5:760. Available online: http://www.asvide.com/article/view/27320
- Celik M, Yuksel UC. Final result. Asvide 2018;5:761. Available online: http://www.asvide.com/article/view/27321
- Mwipatayi BP, Picardo A, Masilonyane-Jones TV, et al. Incidence and prognosis of vascular complications after transcatheter aortic valve implantation. J Vasc Surg 2013;58:1028-36.e1. [Crossref] [PubMed]
- Olasińska-Wiśniewska A, Grygier M, Lesiak M, et al. Femoral artery anatomy-tailored approach in transcatheter aortic valve implantation. Postepy Kardiol Interwencyjnej 2017;13:150-6. [Crossref] [PubMed]
- Barbash IM, Barbanti M, Webb J, et al. Comparison of vascular closure devices for access site closure after transfemoral aortic valve implantation. Eur Heart J 2015;36:3370-9. [Crossref] [PubMed]
- Piffaretti G, Mariscalco G, Tozzi M, et al. Predictive factors of complications after surgical repair of iatrogenic femoral pseudoaneurysms. World J Surg 2011;35:911-6. [Crossref] [PubMed]
Cite this article as: Celik M, Yuksel UC. Successful percutaneous treatment of late-onset femoral pseudoaneurysm after transcatheter aortic valve implantation procedure. J Xiangya Med 2018;3:35.


