
Diagnosis and staging of locally advanced non-small cell lung cancer
Introduction
According to the recent update of the staging of non-small cell lung cancer (NSCLC) by the International Association on the Study of Lung Cancer (IASLC), T4 classifier encompasses rather different entities: a tumour larger than 7 cm; a tumour with a satellite neoplastic nodule in another lobe of the ipsilateral lung, a tumour involving the vertebral body, great vessels, heart, mediastinum, trachea, esophagus, recurrent laryngeal nerve and diaphragm (that was upstaged in the latest version of the TNM from T3) (1). Although a detailed survival sub-analysis of different T4 subcategories was not possible due to the relatively small amount of data, a slightly worse prognosis has been observed in patients with a second nodule in a different lobe of the same lung.
Farjah et al. (2) investigated the trends in treatment of T4 NSCLC in the last decades finding out that, among 13,000 patients T4-NSCLC patients taken from the Surveillance, Epidemiology and End Result program (SEER) database, just 9% received surgery which is reserved for highly selected patients. Moreover, evidences regarding outcomes of resected T4 are poor and often retrospective analysis of a single institution experience (3); nevertheless, long term outcomes of T4 patients is generally dismal, relying mainly on N status. Consequently, preoperative staging of T4 tumours plays a key role and it should be carried out thoughtfully and carefully. As a consequence, T4 NSCLC staging may require several steps according to the anatomical and pathological situation; furthermore a proper management of the preoperative workup showed to have a direct impact on survival (4). The initial evaluation relies upon an accurate clinical evaluation of the patient, a detailed medical history including possible risk factors, a computed tomography (CT) with contrast and a 2-(18) fluoro-2-deoxy-D-glucose (FDG-PET)-CT (5).
Recently, magnetic resonance imaging (MRI) and PET-MRI have been proposed to have a role in the diagnosis of lung cancer, but benefits compared with CT have still not been demonstrated in prospective studies (6) and their routinary use is currently left for selected patients.
Functional assessment
Surgery of T4 NSCLC might require extended procedures which account for not only resection of surrounding structures, but also large parts of lung parenchyma up to complete pneumonectomy. It is therefore of paramount importance a detailed evaluation of the pulmonary and cardiac function prior to any planned treatment. It is mandatory that such factors as life expectancy, tumor stage, performance status, and comorbidities should be taken into account.
Risk of cardiovascular complication after thoracic surgery is about 2–3% (7) and a cardiological preoperative evaluation is therefore needed. Not all the patients should undergo invasive cardiological tests, but only those with a higher risk of complication which might be selected and stratified according to the presence of comorbities or according their medical history (8,9).
Pulmonary function should be evaluated using both pulmonary function tests (PFTs) and diffusing capacity for carbon monoxide (DLCO). Forced expiratory volume in the first second (FEV1) and DLCO showed to be strictly related with morbidity and mortality in particular a preoperative FEV1 lower than 30% and more than 60% were related to and incidence of respiratory morbidity of 43% and 12% respectively (10), while a preoperative DLCO lower than 60% was associated to a 40% pulmonary morbidity and up to 25% of postoperative mortality (11). Interestingly, up to 40% of patients with a preoperative FEV1 higher than 80% has a DLCO lower than 80% and among them the 7% has a postoperative DLCO lower than 40%; DLCO should therefore be always investigated regardless the values of FEV1 (12,13). Together with preoperative values, predicted postoperative (PPO) FEV1 and DLCO can be calculated and they are very sensitive prognostic factors (9,14).
In this cohort of patients an induction therapy is often performed before surgery. Some authors reported a detrimental effect of induction treatment on pulmonary function; in particular DLCO seems to be the most affected parameter (15), even if the long term effect is not clear (16). Consequently, PFTs and DLCO tests should be repeated after neoadjuvant therapy.
In patients undergoing a pneumonectomy, lung function is usually more severely impaired and functional reserve is decreased as well (17,18); consequently, a more careful evaluation of lung and cardiac function is mandatory in the work up of this kind of patients (19). Brunelli and colleagues (18) suggest a routinary a cardiopulmonary exercise test (CPET) before pneumonectomy regardless PFTs and DLCO preoperative and PPO data. Concurrently, echocardiography has been advocated as necessary in the perioperative management of pneumonectomy to evaluate right ventricular function for the risk of developing or worsen pulmonary hypertension (20).
Diagnosis of T category
Diagnostic tests should aim to maximize the yield for both diagnosis and staging avoiding unnecessary invasive tests (21). In large and centrally located NSCLC, in particular with previous episodes of haemoptysis, sputum cytology could be the first diagnostic step in with sensitivity and specificity ranging from 42% to 97% and from 68% to 100% respectively. Nevertheless, this results are strongly influenced by the position of the cancer and the number of specimen collected (22); as a consequence, sputum cytology might be performed, but a negative results required further investigation (21). In case of centrally located masses, bronchoscopy is the procedure of choice to yield specimen either for histologic or cytological examination; overall sensitivity is 88% (21), but it has been reported to be 74%, 48% and 59% for forceps biopsy, washing and brushing respectively. Furthermore, an endobronchial needle aspiration can increase sensitivity of bronchoscopy (23). In case of peripheral lesions, data are less encouraging with an overall sensitivity of 78% and transbronchial needle aspiration seems to be the most sensitive tool in this setting (65%) (22). Nonetheless, sensitivity might be increased by the use of fluoroscopy (24), by a larger number of specimen (25) and by larger tumor diameter (in particular if larger than 2 cm) (26,27). Radial- endobronchial ultrasound (r-EBUS) and electromagnetic navigation (EMN) are relatively new techniques that might increase sensitivity of endoscopic procedures for peripheral nodules. In a meta-analysis, r-EBUS showed to have a sensitivity of 73% and specificity of 100% for peripheral nodules, with higher diagnostic yield for lesion larger than 2 cm (28); additionally, a single centre prospective study showed a diagnostic yield rate of 74% for peripheral nodules (29). Concurrently, a combined use of EBUS and EMN might lead to an interesting diagnostic yield of 93% (30). Nonetheless, transthoracic needle aspiration (TTNA) is currently considered the gold standard for peripheral NSCLC with a sensitivity of 90% (21); CT guided TTNA seems to lead to better results in terms of sensitivity rather than fluoroscopy (92% versus 88%) (22). Moreover, the use of a needle core biopsy seems to bring some advantages compared to fine needle aspiration (FNA); as a matter of fact, despite similar sensitivity for malignancies, it can reach better results in defining non-malignant lesion and to yield enough tissue for genetic and mutation analysis. Importantly, TTNA has a relatively high rate of false negative results (31) and has a moderate complication rate (32).
Superior vena cava (SVC) involvement
Lung cancer invading SVC are quite rare accounting for less than 1% of operable patients. These tumors are often centrally located, possibly requiring pneumonectomy, and might infiltrate the phrenic nerve, which therefore must be sacrificed in the majority of cases.
In the preoperative management of NSCLC involving SVC extension of the infiltration can be studied with cavography with simultaneous injection of contrast agent from both upper limbs, even if this technique might be heavily biased by the presence of overlaying structures which might results in inconclusive images (33,34). Recently, CT angiography (CTA) and magnetic resonance angiography (MRA) has been proposed to assess vessel involvement (Figure 1). Both CTA and MRA showed a higher sensibility compared to cavography in discriminating different anatomical structures and eventually extent of vascular invasion (33); interestingly, some authors reported a similar sensibility both for un-enhanced and enhanced MRA, which might further reduce invasiveness of the test (35,36). Additionally, Ohno and colleagues (37) report a better quality of radiological images with the use of electrocardiographically (ECG)-triggered MRA. Lastly, a echocardiography is mandatory to exclude the presence of thrombus in the right atrium (34).
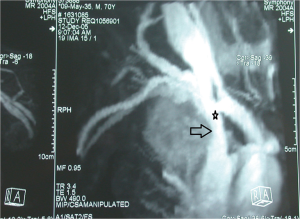
Tracheal and carinal involvement
Bronchoscopy is fundamental in the work up of airways involvement. Mitchell and colleagues (38) suggest the preoperative the use of rigid bronchoscopy not only to verify feasibility of the resection, but also to plan it in details. Furthermore, preoperative rigid bronchoscopy could be useful to perform an endoscopic debulking to prevent the development of postoperative pneumonia.
Aorta involvement
In one large European multicentre study (39) and a single institution paper (40) of NSCLC invading thoracic aorta, authors suggest that possible aortic infiltration should be investigated both by contrasted CT and MRI, which can adequately define particular of vascular invasion. On the other hand, none of the authors suggest the routinely use of angiographic tests.
Left atrial involvement
Intrapericardial invasion of the heart by NSCLC is generally considered a contra-indication for surgery; nevertheless, left atrium might be involved either by a direct diffusion of the tumor or by a neoplastic thrombus arising from the pulmonary vein. Echocardiography is the first-level test to assess heart involvement; in particular, transesophageal echocardiography can be used both in the preoperative workup and as intraoperative monitoring to verify haemodynamic impact of left atrium clamping, as described by Stella colleagues in a series of 31 consecutive cases (41). Beside echocardiography, Galvaing et al. (42) suggests a routinary use of preoperative cardiac MRI to assess a possible infiltration of interatrial septa. In case of heart involvement, a cardio-pulmonary bypass (CPB) should be used, even if many authors reported the use of a clamp-and-suture technique.
Superior sulcus tumors
Superior sulcus tumor might be considered as T3 or T4 according to the extension to the vertebrae, the subclavian vessels or the brachial plexus beside the chest wall. Although T4 are not uniformly considered operable, Waseda and colleagues (43) report similar long term outcomes compared with T3 in experienced hands. Preoperative staging should always consider MRI for a better definition of the involvement of the surrounding structures and better define the surgical approach.
Diagnosis of N category
N status should probably be considered the most important prognostic factors in the management of T4 tumors. As a matter of fact, beside the technical evaluation of surgical resectability of the T component, lymph-node involvement is crucial to decide the better therapeutic path to follow. In a multicentre study, Reed et al. (44) confirmed the utility of PET scan, in particular for the staging of mediastinal nodes and unexpected metastasis, while Cerfolio et al. (45) suggested that, in case of positivity at PET scan in a mediastinal lymph-node station or a distant organ, further analysis and possibly histological biopsy should be always performed to confirm the positivity. The routinely use of PET scan is strongly recommended also by the National Comprehensive Cancer Network (NCCN) and the European Society of Thoracic Surgeons (ESTS) (46) guidelines for its higher sensitivity (80–90%) and specificity (85–95%), compared with other radiological techniques. Concurrently, a recent meta-analysis (47) comparing PET-CT and diffusion-weighted MRI finding a better sensitivity for the latter; nevertheless, to date, no high grade evidences justify the routinary use of Magnetic Resonance for the specific diagnosis of N component. In addition to this, ESTS guidelines (46) pointed out some exception in the solely use of PET-CT for the staging of mediastinal lymph-nodes, which are: tumors larger than 3 cm, suspected N1 and centrally located tumour without suspected nodes on CT or PET scan. It is therefore clear that in case of a T4 tumour, the negative predictive value (NPV) of PET-CT in the investigation of mediastinal lymph node should be always taken with caution and further analysis should be performed, also in case of a completely negative preoperative radiological work up.
EBUS techniques and endoscopic ultrasounds (EUS), are commonly used by endoscopists and thoracic surgeons and they allow the exploration of almost all mediastinal lymph-node stations (48), except station 5 and 6 which are not recommended to be explored with these techniques. Trials and meta-analysis (49-54) investigated the specificity and sensitivity of each technique, finding the best results with the combined use of EUS and EBUS (sensitivity of 83% to 94% for mediastinal staging of lung cancer), reducing the need for further invasive diagnostic procedures (46,55,56). Surgical approaches for lymph-node staging account for video-assisted mediastinoscopy (VAM), video-assisted mediastinoscopic lymph-adenectomy (VAMLA) (57) transcervical extended mediastinal lymphadenectomy (TEMLA) (58) and VATS; although they are standardized and safe procedures, they are plagued by a higher incidence of morbidity compared to endoscopic procedures. VAM is the most diffuse surgical procedure for the evaluation of pathological mediastinal lymph-nodes; it allows a histological sampling of all paratracheal stations, subcarinal and the most proximal hilar stations; conversely, stations 5, 6, 8, 9 are not reachable (46). The use of VAMLA and TEMLA have been described by several institutions, but their use it is neither largely diffuse nor even suggested by ESTS guidelines as morbidity and mortality rates are significantly higher than VAM. On the other hand, investigation of station 5 and 6 might require different approaches. VAMLA and TEMLA showed the highest NPV, which reaches an impressive 98.7% (46). Furthermore, also VATS and anterior mediastinotomy (Chamberlain procedure) can be performed as diagnostic procedures.
Interestingly, Mitchell and his colleagues advocated for the use of mediastinoscopy just before surgical resection of tumors invading trachea, as it allows not only a correct analysis of nodal status prior to a complex surgical procedure, but also it can free the trachea by creating the pretracheal space (38).
Induction therapy is frequently requested for locally advanced NSCLC. Restaging is therefore mandatory after neoadjuvant treatment to re-assess the nodal status and consequently give the proper indication (59). CT scan and PET-CT lose part of their sensitivity, specificity and NPV in the restaging after induction therapy and are therefore not fully reliable leading to over- or down-staging (60). As a matter of fact, EBUS and EUS should be used as first line procedures to assess possible persistence of disease, but their sensitivity and false negative rate suggest the use of a surgical staging in case of negative results (59,61,62). Although mediastinoscopy in naïve patients is feasible and safe even after induction therapy (63), remediastinoscopy is a feasible, but challenging procedures that should be performed with caution in experienced centres (59,61).
Diagnosis of M category
Presence of distant metastasis is a contraindication for surgical resection in T4 NSCLC and they should be investigated prior to any invasive procedure. In a meta-analysis investigating accuracy of 18FDG PET-CT in detecting lung cancer metastasis, Li and colleagues (64) reported a sensitivity and specificity of 92% and 97% respectively. Concurrently, two meta-analysis (65,66) comparing PET-CT and MRI found similar results in terms of sensitivity and specificity suggesting that a combined use might improve the preoperative staging. Nevertheless, due to the baseline cerebral activity, PET-CT is not suitable to a correct staging of the brain. In stage III or IV tumors, American College of Chest Physicians (ACCP) guidelines suggest to investigate the brain using MRI or CT scan even in patients with no clinical sign of cerebral involvement (5). Concurrently, a single-institution Korean study suggested that evaluation of the brain might be suggested only in patients with higher risk factors (67).
Summary
T4 tumors represent a rare presentation of NSCLC and they can be considered a double challenge for thoracic surgeons: they imply a correct staging and a correct evaluation of technical feasibility of surgical resection. In fact, both N status and radicality of the intervention are two fundamental prognostic factors that strongly affect long term results. In order to choose the correct treatment, multidisciplinary discussion is mandatory and should include thoracic surgeons, oncologists, radiation oncologists and interventional pneumonologists. Moreover, three main points should be always followed in the management of T4 NSCLC: firstly, surgical treatment of T4 tumors usually required skills and experience and they should be referred to higher volume institutions with an acceptable expertise (9); secondly, CT scan, PET-CT, and any further analysis should be performed on a case-by-case fashion according to anatomic and oncologic features; lastly, pulmonary and cardiac functionality should be investigated in details.
In conclusion, management of T4 NSCLC require a multidisciplinary and multimodality work-up in order to decide the better and more correct treatment for the disease.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Fabio Davoli, Sai Yendamuri) for the series “Extended Pulmonary Resections for T4 Non-Small-Cell-Lung-Cancer” published in Journal of Xiangya Medicine. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jxym.2018.07.03). The series “Extended Pulmonary Resections for T4 Non-Small-Cell-Lung-Cancer” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Rami-Porta R, Bolejack V, Crowley J, et al. The IASLC Lung Cancer Staging Project: Proposals for the Revisions of the T Descriptors in the Forthcoming Eighth Edition of the TNM Classification for Lung Cancer. J Thorac Oncol 2015;10:990-1003.
- Farjah F, Wood DE, Varghese TK, et al. Trends in the operative management and outcomes of T4 lung cancer. Ann Thorac Surg 2008;86:368-74. [Crossref] [PubMed]
- Yildizeli B, Dartevelle PG, Fadel E, et al. Results of primary surgery with T4 non-small cell lung cancer during a 25-year period in a single center: the benefit is worth the risk. Ann Thorac Surg 2008;86:1065-75; discussion 1074-5. [Crossref] [PubMed]
- Farjah F, Flum DR, Ramsey SD, et al. Multi-modality mediastinal staging for lung cancer among medicare beneficiaries. J Thorac Oncol 2009;4:355-63. [Crossref] [PubMed]
- Silvestri GA, Gonzalez AV, Jantz MA, et al. Methods for staging non-small cell lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:e211S-50S.
- Ohno Y, Koyama H, Lee HY, et al. Magnetic Resonance Imaging (MRI) and Positron Emission Tomography (PET)/MRI for Lung Cancer Staging. J Thorac Imaging 2016;31:215-27. [Crossref] [PubMed]
- Brunelli A, Cassivi SD, Fibla J, et al. External validation of the recalibrated thoracic revised cardiac risk index for predicting the risk of major cardiac complications after lung resection. Ann Thorac Surg 2011;92:445-8. [Crossref] [PubMed]
- Lee TH, Marcantonio ER, Mangione CM, et al. Derivation and prospective validation of a simple index for prediction of cardiac risk of major noncardiac surgery. Circulation 1999;100:1043-9. [Crossref] [PubMed]
- Brunelli A, Kim AW, Berger KI, et al. Physiologic evaluation of the patient with lung cancer being considered for resectional surgery: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:e166S-90S.
- Berry MF, Villamizar-Ortiz NR, Tong BC, et al. Pulmonary function tests do not predict pulmonary complications after thoracoscopic lobectomy. Ann Thorac Surg 2010;89:1044-51; discussion 51-2. [Crossref] [PubMed]
- Ferguson MK, Little L, Rizzo L, et al. Diffusing capacity predicts morbidity and mortality after pulmonary resection. J Thorac Cardiovasc Surg 1988;96:894-900. [PubMed]
- Brunelli A, Refai MA, Salati M, et al. Carbon monoxide lung diffusion capacity improves risk stratification in patients without airflow limitation: evidence for systematic measurement before lung resection. Eur J Cardiothorac Surg 2006;29:567-70. [Crossref] [PubMed]
- Ferguson MK, Vigneswaran WT. Diffusing capacity predicts morbidity after lung resection in patients without obstructive lung disease. Ann Thorac Surg 2008;85:1158-64; discussion 64-5. [Crossref] [PubMed]
- Ferguson MK, Reeder LB, Mick R. Optimizing selection of patients for major lung resection. J Thorac Cardiovasc Surg 1995;109:275-81; discussion 81-3. [Crossref] [PubMed]
- Rivera MP, Detterbeck FC, Socinski MA, et al. Impact of preoperative chemotherapy on pulmonary function tests in resectable early-stage non-small cell lung cancer. Chest 2009;135:1588-95. [Crossref] [PubMed]
- Margaritora S, Cesario A, Cusumano G, et al. Is pulmonary function damaged by neoadjuvant lung cancer therapy? A comprehensive serial time-trend analysis of pulmonary function after induction radiochemotherapy plus surgery. J Thorac Cardiovasc Surg 2010;139:1457-63. [Crossref] [PubMed]
- Melloul E, Egger B, Krueger T, et al. Mortality, complications and loss of pulmonary function after pneumonectomy vs. sleeve lobectomy in patients younger and older than 70 years. Interact Cardiovasc Thorac Surg 2008;7:986-9. [Crossref] [PubMed]
- Brunelli A, Xiumé F, Refai M, et al. Evaluation of expiratory volume, diffusion capacity, and exercise tolerance following major lung resection: a prospective follow-up analysis. Chest 2007;131:141-7. [Crossref] [PubMed]
- Brunelli A. Preoperative functional workup for patients with advanced lung cancer. J Thorac Dis 2016;8:S840-8. [Crossref] [PubMed]
- Amar D, Burt ME, Roistacher N, et al. Value of perioperative Doppler echocardiography in patients undergoing major lung resection. Ann Thorac Surg 1996;61:516-20. [Crossref] [PubMed]
- Rivera MP, Mehta AC, Wahidi MM. Establishing the diagnosis of lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:e142S-65S.
- Schreiber G, McCrory DC. Performance characteristics of different modalities for diagnosis of suspected lung cancer: summary of published evidence. Chest 2003;123:115S-28S. [Crossref] [PubMed]
- Dasgupta A, Jain P, Minai OA, et al. Utility of transbronchial needle aspiration in the diagnosis of endobronchial lesions. Chest 1999;115:1237-41. [Crossref] [PubMed]
- Cox ID, Bagg LR, Russell NJ, et al. Relationship of radiologic position to the diagnostic yield of fiberoptic bronchoscopy in bronchial carcinoma. Chest 1984;85:519-22. [Crossref] [PubMed]
- Eberhardt R, Anantham D, Ernst A, et al. Multimodality bronchoscopic diagnosis of peripheral lung lesions: a randomized controlled trial. Am J Respir Crit Care Med 2007;176:36-41. [Crossref] [PubMed]
- Trkanjec JT, Peros-Golubicić T, Grozdek D, et al. The role of transbronchial lung biopsy in the diagnosis of solitary pulmonary nodule. Coll Antropol 2003;27:669-75. [PubMed]
- Bandoh S, Fujita J, Tojo Y, et al. Diagnostic accuracy and safety of flexible bronchoscopy with multiplanar reconstruction images and ultrafast Papanicolaou stain: evaluating solitary pulmonary nodules. Chest 2003;124:1985-92. [Crossref] [PubMed]
- Steinfort DP, Khor YH, Manser RL, et al. Radial probe endobronchial ultrasound for the diagnosis of peripheral lung cancer: systematic review and meta-analysis. Eur Respir J 2011;37:902-10. [Crossref] [PubMed]
- Gildea TR, Mazzone PJ, Karnak D, et al. Electromagnetic navigation diagnostic bronchoscopy: a prospective study. Am J Respir Crit Care Med 2006;174:982-9. [Crossref] [PubMed]
- Mahajan AK, Patel S, Hogarth DK, et al. Electromagnetic navigational bronchoscopy: an effective and safe approach to diagnose peripheral lung lesions unreachable by conventional bronchoscopy in high-risk patients. J Bronchology Interv Pulmonol 2011;18:133-7. [Crossref] [PubMed]
- Zarbo RJ, Fenoglio-Preiser CM. Interinstitutional database for comparison of performance in lung fine-needle aspiration cytology. A College of American Pathologists Q-Probe Study of 5264 cases with histologic correlation. Arch Pathol Lab Med 1992;116:463-70. [PubMed]
- Wiener RS, Schwartz LM, Woloshin S, et al. Population-based risk for complications after transthoracic needle lung biopsy of a pulmonary nodule: an analysis of discharge records. Ann Intern Med 2011;155:137-44. [Crossref] [PubMed]
- Eren S, Karaman A, Okur A. The superior vena cava syndrome caused by malignant disease. Imaging with multi-detector row CT. Eur J Radiol 2006;59:93-103. [Crossref] [PubMed]
- Dartevelle PG, Mitilian D, Fadel E. Extended surgery for T4 lung cancer: a 30 years' experience. Gen Thorac Cardiovasc Surg 2017;65:321-8. [Crossref] [PubMed]
- Wang L, Lv P, Yang S, et al. Assessment of thoracic vasculature in patients with central bronchogenic carcinoma by unenhanced magnetic resonance angiography: comparison between 2D free-breathing TrueFISP, 2D breath-hold TrueFISP and 3D respiratory-triggered SPACE. J Thorac Dis 2017;9:1624-33. [Crossref] [PubMed]
- Ohno Y, Nishio M, Koyama H, et al. Journal Club: Comparison of assessment of preoperative pulmonary vasculature in patients with non-small cell lung cancer by non-contrast- and 4D contrast-enhanced 3-T MR angiography and contrast-enhanced 64-MDCT. AJR Am J Roentgenol 2014;202:493-506. [Crossref] [PubMed]
- Ohno Y, Adachi S, Motoyama A, et al. Multiphase ECG-triggered 3D contrast-enhanced MR angiography: utility for evaluation of hilar and mediastinal invasion of bronchogenic carcinoma. J Magn Reson Imaging 2001;13:215-24. [Crossref] [PubMed]
- Mitchell JD, Mathisen DJ, Wright CD, et al. Resection for bronchogenic carcinoma involving the carina: long-term results and effect of nodal status on outcome. J Thorac Cardiovasc Surg 2001;121:465-71. [Crossref] [PubMed]
- Marulli G, Rendina EA, Klepetko W, et al. Surgery for T4 lung cancer invading the thoracic aorta: Do we push the limits? J Surg Oncol 2017;116:1141-9. [Crossref] [PubMed]
- Misthos P, Papagiannakis G, Kokotsakis J, et al. Surgical management of lung cancer invading the aorta or the superior vena cava. Lung Cancer 2007;56:223-7. [Crossref] [PubMed]
- Stella F, Dell'Amore A, Caroli G, et al. Surgical results and long-term follow-up of T(4)-non-small cell lung cancer invading the left atrium or the intrapericardial base of the pulmonary veins. Interact Cardiovasc Thorac Surg 2012;14:415-9. [Crossref] [PubMed]
- Galvaing G, Tardy MM, Cassagnes L, et al. Left atrial resection for T4 lung cancer without cardiopulmonary bypass: technical aspects and outcomes. Ann Thorac Surg 2014;97:1708-13. [Crossref] [PubMed]
- Waseda R, Klikovits T, Hoda MA, et al. Trimodality therapy for Pancoast tumors: T4 is not a contraindication to radical surgery. J Surg Oncol 2017;116:227-35. [Crossref] [PubMed]
- Reed CE, Harpole DH, Posther KE, et al. Results of the American College of Surgeons Oncology Group Z0050 trial: the utility of positron emission tomography in staging potentially operable non-small cell lung cancer. J Thorac Cardiovasc Surg 2003;126:1943-51. [Crossref] [PubMed]
- Cerfolio RJ, Ojha B, Bryant AS, et al. The role of FDG-PET scan in staging patients with nonsmall cell carcinoma. Ann Thorac Surg 2003;76:861-6. [Crossref] [PubMed]
- De Leyn P, Dooms C, Kuzdzal J, et al. Revised ESTS guidelines for preoperative mediastinal lymph node staging for non-small-cell lung cancer. Eur J Cardiothorac Surg 2014;45:787-98. [Crossref] [PubMed]
- Wu LM, Xu JR, Gu HY, et al. Preoperative mediastinal and hilar nodal staging with diffusion-weighted magnetic resonance imaging and fluorodeoxyglucose positron emission tomography/computed tomography in patients with non-small-cell lung cancer: which is better? J Surg Res 2012;178:304-14. [Crossref] [PubMed]
- Rusch VW, Asamura H, Watanabe H, et al. The IASLC lung cancer staging project: a proposal for a new international lymph node map in the forthcoming seventh edition of the TNM classification for lung cancer. J Thorac Oncol 2009;4:568-77.
- Liberman M, Sampalis J, Duranceau A, et al. Endosonographic mediastinal lymph node staging of lung cancer. Chest 2014;146:389-97. [Crossref] [PubMed]
- Micames CG, McCrory DC, Pavey DA, et al. Endoscopic ultrasound-guided fine-needle aspiration for non-small cell lung cancer staging: A systematic review and metaanalysis. Chest 2007;131:539-48. [Crossref] [PubMed]
- Gu P, Zhao YZ, Jiang LY, et al. Endobronchial ultrasound-guided transbronchial needle aspiration for staging of lung cancer: a systematic review and meta-analysis. Eur J Cancer 2009;45:1389-96. [Crossref] [PubMed]
- Adams K, Shah PL, Edmonds L, et al. Test performance of endobronchial ultrasound and transbronchial needle aspiration biopsy for mediastinal staging in patients with lung cancer: systematic review and meta-analysis. Thorax 2009;64:757-62. [Crossref] [PubMed]
- Chandra S, Nehra M, Agarwal D, et al. Diagnostic accuracy of endobronchial ultrasound-guided transbronchial needle biopsy in mediastinal lymphadenopathy: a systematic review and meta-analysis. Respir Care 2012;57:384-91. [PubMed]
- Zhang R, Ying K, Shi L, et al. Combined endobronchial and endoscopic ultrasound-guided fine needle aspiration for mediastinal lymph node staging of lung cancer: a meta-analysis. Eur J Cancer 2013;49:1860-7. [Crossref] [PubMed]
- Tournoy KG, De Ryck F, Vanwalleghem LR, et al. Endoscopic ultrasound reduces surgical mediastinal staging in lung cancer: a randomized trial. Am J Respir Crit Care Med 2008;177:531-5. [Crossref] [PubMed]
- Vilmann P, Clementsen PF, Colella S, et al. Combined endobronchial and oesophageal endosonography for the diagnosis and staging of lung cancer. European Society of Gastrointestinal Endoscopy (ESGE) Guideline, in cooperation with the European Respiratory Society (ERS) and the European Society of Thoracic Surgeons (ESTS). Eur Respir J 2015;46:40-60. [Crossref] [PubMed]
- Hürtgen M, Friedel G, Toomes H, et al. Radical video-assisted mediastinoscopic lymphadenectomy (VAMLA)--technique and first results. Eur J Cardiothorac Surg 2002;21:348-51. [Crossref] [PubMed]
- Kuzdzał J, Zieliński M, Papla B, et al. Transcervical extended mediastinal lymphadenectomy--the new operative technique and early results in lung cancer staging. Eur J Cardiothorac Surg 2005;27:384-90; discussion 90. [Crossref] [PubMed]
- Rami-Porta R, Call S. Invasive staging of mediastinal lymph nodes: mediastinoscopy and remediastinoscopy. Thorac Surg Clin 2012;22:177-89. [Crossref] [PubMed]
- de Cabanyes Candela S, Detterbeck FC. A systematic review of restaging after induction therapy for stage IIIa lung cancer: prediction of pathologic stage. J Thorac Oncol 2010;5:389-98. [Crossref] [PubMed]
- von Bartheld MB, Versteegh MI, Braun J, et al. Transesophageal ultrasound-guided fine-needle aspiration for the mediastinal restaging of non-small cell lung cancer. J Thorac Oncol 2011;6:1510-5. [Crossref] [PubMed]
- De Leyn P, Lardinois D, Van Schil PE, et al. ESTS guidelines for preoperative lymph node staging for non-small cell lung cancer. Eur J Cardiothorac Surg 2007;32:1-8. [Crossref] [PubMed]
- Lardinois D, Schallberger A, Betticher D, et al. Postinduction video-mediastinoscopy is as accurate and safe as video-mediastinoscopy in patients without pretreatment for potentially operable non-small cell lung cancer. Ann Thorac Surg 2003;75:1102-6. [Crossref] [PubMed]
- Li J, Xu W, Kong F, et al. Meta-analysis: accuracy of 18FDG PET-CT for distant metastasis staging in lung cancer patients. Surg Oncol 2013;22:151-5. [Crossref] [PubMed]
- Ohno Y, Koyama H, Onishi Y, et al. Non-small cell lung cancer: whole-body MR examination for M-stage assessment--utility for whole-body diffusion-weighted imaging compared with integrated FDG PET/CT. Radiology 2008;248:643-54. [Crossref] [PubMed]
- Xu GZ, Li CY, Zhao L, et al. Comparison of FDG whole-body PET/CT and gadolinium-enhanced whole-body MRI for distant malignancies in patients with malignant tumors: a meta-analysis. Ann Oncol 2013;24:96-101. [Crossref] [PubMed]
- Na II, Lee TH, Choe DH, et al. A diagnostic model to detect silent brain metastases in patients with non-small cell lung cancer. Eur J Cancer 2008;44:2411-7. [Crossref] [PubMed]
Cite this article as: Viti A, Bertoglio P, Terzi AC. Diagnosis and staging of locally advanced non-small cell lung cancer. J Xiangya Med 2018;3:32.


