
Predictors of post-operative atrial fibrillation in patients undergoing cardiac surgery: the potential role of epicardial adipose tissue
Introduction
Post-operative atrial fibrillation (POAF) is reported in 15% to 40% of patients in the early post-operative period after coronary artery bypass graft (CABG), and in 40% to 50% of patients after isolated valve surgery or combined CABG/valve surgery (1-3). Up to 20% of patients may not revert to sinus rhythm within 24 hours of POAF onset in patients without previous history of atrial fibrillation (AF) (4). Moreover, POAF is associated with increased duration of hospitalisation, healthcare costs, and incidence of stroke or need for a permanent pacemaker with associated mortality (1,5).
Many cardiovascular risk factors have been associated with AF, however they lack predictive accuracy to identify patients who will develop POAF (6,7). There has been recent interest in the potential of epicardial adipose tissue (EAT) as a marker of both coronary artery disease (CAD) and risk of future AF. Increasing evidence seems to indicate pericardial fat as a metabolically active tissue modulating the adjacent myocardial tissue in paracrine and vasocrine manners with production of pro-atherogenic and pro-inflammatory adipokines (8-10). Several studies including the Framingham Heart Study (11), the Multi-Ethnic Study of Atherosclerosis (12) and others have demonstrated a relationship of increased pericardial fat volume with CAD, atherosclerosis, progression of coronary plaque burden and AF (13-15).
In the context of cardiac surgery, inflammation due to cardiopulmonary bypass, ischemia and oxidative stress have been implicated in the development of POAF (16-18). A recent study showed that pericardial fat volume measured on computed tomography (CT) is strongly associated with AF after CABG, independent of traditional risk factors (19). With the advent of non-invasive imaging techniques, it would be useful to predict POAF in patients undergoing cardiac surgery utilizing pre-operative characteristics on routine investigations. Such prediction would allow for pre-emptive anti-arrhythmic therapy that could potentially decrease AF occurrence post-operatively. Therefore, our aim was to investigate clinical predictors of POAF, including whether EAT improves risk prediction.
Methods
The study was in compliance with the principles outlined in the Declaration of Helsinki and approved by Institutional Ethics Committee. Fifty-six consecutive patients who underwent first-time aortic valve replacement (AVR) or on-pump CABG with or without AVR during 2013 to 2015 were included in this study. Patients who had undergone thoracic CT scan in the previous six months were included in the study from the Mount Hospital Cardiothoracic Database. Consent was gained after the patients’ procedures for inclusion of their data in this study. Patients with permanent AF, prior permanent pacemaker or AF ablation were excluded to avoid bias in POAF incidence. Study variables included age, sex, body mass index (BMI), smoking status, medications, comorbidities and pre- and post-operative laboratory tests. POAF was defined as any documented episode of AF post-operatively, both treated and untreated, during hospital stay. Echocardiogram and coronary angiogram performed within six months prior to the patients’ surgery was included in the data collection.
EAT measurements
CT scans performed within six months prior to the surgery included a range of different scan protocols performed for varying clinical indications, hence, the study included both contrast and non-contrast scans, and scans with and without cardiac ECG gating. A consultant radiologist, blinded to the POAF status of the subjects, measured the EAT parameters in this study. EAT measurements can substantially differ between contrast-enhanced and non-contrast CT, but can be reconciled by threshold modification (20). Therefore, an upper threshold on fat volume of −15 Hounsfield unit (HU) for contrast CT was used and −45 HU threshold for non-contrast CT images (20). The EAT thickness measurements were made using protocol described by Yorgun et al. (21). Multiplanar reconstructions of the axial raw data were created in the horizontal long axis (four-chamber) view, and ventricular short axis and at the basal, mid ventricular and apical levels on a workstation (Advantage Windows GE Healthcare) with a slice thickness of 3 mm. EAT was defined as the adipose tissue between the myocardium and the epicardium. EAT thickness measurements were performed from seven short-axis view segments (Figure 1) including right ventricular (RV) anterior free wall superior, RV anterior free wall inferior, RV superior wall, RV diaphragmatic wall, superior interventricular (IV) groove, left ventricular (LV) lateral wall. The mean of measurements taken at those levels (basal, mid, apical) was used for analyses.
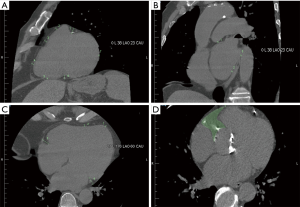
The left atrium (LA) short-axis view (Figure 1B) was reconstructed as a plane perpendicular to the long axis of standard two- and four-chamber views of LA at the level of the mid LA. In this short-axis view, the periatrial EAT thickness was measured (in mm) as the shortest distance between the mid LA wall and the esophagus (LA-Eso), main pulmonary artery (LA-PA), and descending thoracic aorta (LA-Desc Ao).
On the horizontal long axis (four-chamber) view (Figure 1C), EAT was measured from four segments including LV apex, RV apex and peri-coronary areas [left atrioventricular (AV) groove and right AV groove]. Total EAT thickness was calculated as the sum of EAT thickness measurements from eleven segments including the apical four chamber views and the short-axis views
The EAT volumetric measurements were made using protocol described by Saremi et al. (22). The right coronary artery fat pocket area (RCA-FP) (Figure 1D) was defined as the area of EAT at the level of RCA origin located between the right atrium, ascending aorta, and RV outflow tract measured in the axial plane. The anterior border of the RCA-FP was limited by the pericardial line extending between its closest points with the anterior margins of the RA and right ventricle outflow tract.
Statistical analysis
Continuous variables are presented as mean values ± standard deviation (SD) for normally distributed data, or median and interquartile ranges for non-parametric data. Univariate analyses were performed on continuous variables using Mann-Whitney U test. Categorical variables are presented as percentages and differences between nominal variables were analyzed using the chi-square test. Logistic regression analysis was performed for the level of significance of risk factors and between-group relationships. A multivariate logistic regression model was performed to estimate the odds ratio (OR) of POAF while controlling for potential confounding variables and/or known risk factors of AF, but not including those variables in the CHA2DS2-Vasc or EuroSCORE II, to avoid interdependence (BMI, valvular heart disease, diabetes mellitus, LV-EF, pre-operative creatinine, pre-operative hemoglobin, statin and aspirin use). P values <0.05 were considered statistically significant. Statistical analyses were performed using SPSS® statistical software (version 23; SPSS Inc., Chicago, IL, USA).
POAF prediction scoring system
Using the most significantly associated variables found in this study (CHA2DS2-Vasc score, LA diameter, EUROScore II, and EAT) we developed a POAF prediction scoring system (Table 1) using cut-off values of twice the standard deviation for each variable. For the CHA2DS2-Vasc score, the cut-off score was allocated as ≥2 as this normally represents high-risk patients in whom anticoagulation is recommended (23). Each patient’s POAF prediction score comprised of the sum of their CHA2DS2-Vasc score plus one point for every variable that exceeded the cut-off score.
Table 1
| Prediction score for each risk factor | 1 point if at least |
|---|---|
| CHA2DS2-Vasc | 2 |
| EuroSCORE II | 4.8 |
| LA diameter (cm) | 4.8 |
| Sum of RCA-FP and IIG | 11.4 |
Each risk factor predicted atrial fibrillation above the threshold presented in this table. The total score was determined by the sum of the CHA2DS2 -Vasc and one point for every risk factor that was above the threshold. IIG, inferior interventricular groove; LA, left atrium; RCA-FP, right coronary artery fat pocket area.
Results
In the study cohort of 56 patients (79% male), POAF occurred in 48% of patients. Three patients had pre-operative paroxysmal or persistent AF (5%). POAF occurred most commonly on day three post-operation. Twenty-eight patients underwent first-time AVR procedures and 28 patients received CABG with or without AVR. The incidence of POAF was greater in patients undergoing CABG with or without AVR compared to AVR alone (64% vs. 32%, P=0.018).
The clinical characteristics of the study population are shown in Table 2. Of note, the median age was higher in the POAF population (81 years, range: 70.0–84.0 years) than the no-POAF population (76 years, range: 57.5–81.0 years) (P=0.043). Univariate logistic regression model showed CHA2DS2-VASc score [odds ratio (OR): 1.629; 95% CI: 1.086–2.443; P=0.018] and EuroSCORE II (OR: 1.528; 95% CI: 1.006–2.320; P=0.047) to be significantly associated with POAF. Pre-operative investigations including echocardiography and angiography results are outlined in Table 3. Between-group analysis showed left atrial (LA) diameter was significantly associated with POAF compared to no-POAF (4.18±0.74 vs. 3.74±0.36 cm; P=0.047). Patients with POAF had numerically, but not significantly, higher E:E’ ratios (18.00±9.44 vs. 12.81±4.44; P=0.08).
Table 2
| Variable | All patients (n=56) | AVR (n=28) | CABG or CABG + AVR (n=28) | POAF positive (n=27) | POAF negative (n=29) | P value |
|---|---|---|---|---|---|---|
| Age, years | 78 (65.5–82.0) | 76 (58.0–82.0) | 79 (70.3–82.8) | 81 (70.0–84.0) | 76 (57.5–81.0) | 0.043 |
| Male | 44 (79%) | 20 (71%) | 24 (86%) | 21 (78%) | 23 (79%) | 0.889 |
| BMI, kg/m2 | 28.7 (24.4–31.7) | 30.5 (25.4–33.2) | 28.5 (23.0–30.4) | 28.7 (22.6–32.1) | 28.6 (25.0–31.6) | 0.825 |
| Current smoker | 4 (7%) | 2 (7%) | 2 (7%) | 1 (4%) | 3 (10%) | 0.323 |
| Hypertension | 44 (79%) | 20 (71%) | 24 (86%) | 23 (85%) | 21 (72%) | 0.244 |
| Dyslipidaemia | 46 (82%) | 20 (71%) | 26 (93%) | 22 (81%) | 24 (83%) | 0.901 |
| Diabetes | 13 (23%) | 6 (21%) | 7 (25%) | 6 (22%) | 7 (24%) | 0.867 |
| Heart failure | 4 (7%) | 1 (4%) | 3 (11%) | 3 (11%) | 1 (3%) | 0.257 |
| FMHx of premature CAD | 9 (16%) | 3 (11%) | 6 (21%) | 3 (11%) | 6 (21%) | 0.325 |
| Paroxysmal AF | 3 (5%) | 2 (7%) | 1 (4%) | 2 (7%) | 1 (3%) | 0.605 |
| Prior stroke | 1 (2%) | 0 | 1 (4%) | 1 (4%) | 0 | 0.224 |
| Prior TIA | 4 (7%) | 3 (11%) | 1 (4%) | 3 (11%) | 1 (3%) | 0.257 |
| Chronic renal failure | 5 (9%) | 0 | 5 (18%) | 4 (15%) | 1 (3%) | 0.126 |
| PVD | 5 (9%) | 2 (7%) | 3 (11%) | 2 (7%) | 3 (10%) | 0.699 |
| Hypothyroidism | 6 (11%) | 2 (7%) | 4 (14%) | 3 (11%) | 3 (10%) | 0.926 |
| COPD | 1 (2%) | 1 (4%) | 0 | 0 | 1 (3%) | 0.248 |
| Scoring systems | ||||||
| EuroSCORE II, % | 1.63 (0.87–2.61) | 1.33 (0.80–2.42) | 2.11 (1.16–3.47) | 2.23 (1.16–3.70) | 1.26 (0.67–2.13) | 0.006 |
| CHA2DS2-Vasc | 4 [2.0–4.8] | 3 [2–4] | 4 [3–5] | 4 [4–5] | 3.0±1.6 | 0.019 |
| Regular medications | ||||||
| Beta-blockers | 19 (34%) | 5 (18%) | 14 (50%) | 10 (37%) | 9 (31%) | 0.635 |
| Calcium channel blocker | 18 (32%) | 6 (21%) | 12 (43%) | 11 (41%) | 7 (24%) | 0.184 |
| ACEI | 20 (36%) | 7 (25%) | 13 (46%) | 9 (33%) | 11 (38%) | 0.72 |
| ARB | 22 (39%) | 12 (43%) | 10 (36%) | 14 (52%) | 8 (28%) | 0.063 |
| Statins | 45 (80%) | 19 (68%) | 26 (93%) | 23 (85%) | 22 (76%) | 0.38 |
| Aspirin | 33 (59%) | 13 (46%) | 20 (71%) | 17 (63%) | 16 (55%) | 0.554 |
| Amiodarone | 1 (2%) | 1 (4%) | 0 | 1 (4%) | 0 | 0.224 |
ACEI, angiotensin converting enzyme inhibitor; AF, atrial fibrillation; ARB, angiotensin 2 receptor blockers; AVR, aortic valve replacement; BMI, basal metabolic index; CABG, coronary artery bypass grafting; CAD, coronary artery disease; COPD, chronic obstructive pulmonary disease; FMHx, family history; POAF, post-operative atrial fibrillation; PVD, peripheral vascular disease; TIA, transient ischemic attack.
Table 3
| Variable | All patients (n=56) | AVR (n=28) | CABG or CABG + AVR (n=28) | POAF positive (n=27) | POAF negative (n=29) | P value |
|---|---|---|---|---|---|---|
| Echocardiography | ||||||
| LVEF, % | 59.5±12.5 | 63.7±10.8 | 54.8±12.8 | 60.1±13.3 | 64.0±11.7 | 0.628 |
| LA diameter, cm | 4.00±0.64 | 3.87±0.42 | 4.11±0.79 | 4.18±0.74 | 3.74±0.36 | 0.047 |
| LVEDD, cm | 4.50±0.70 | 4.55±0.78 | 4.45±0.63 | 4.38±0.68 | 4.40±0.73 | 0.187 |
| IVS, cm | 1.33±0.23 | 1.33±0.18 | 1.33±0.29 | 1.35±0.26 | 1.30±0.21 | 0.725 |
| LVPW, cm | 1.24±0.20 | 1.28±0.21 | 1.20±0.20 | 1.27±0.18 | 1.30±0.22 | 0.203 |
| E:E’ ratio | 15.41±7.70 | 12.42±3.02 | 18.84±9.95 | 18.00±9.44 | 12.81±4.44 | 0.08 |
| Angiography | ||||||
| Gensini score | 3.25±3.56 | 0.74±0.84 | 5.04±3.68 | 3.76±3.99 | 2.44±2.67 | 0.346 |
| Sullivan score | 15.65±14.75 | 4.97±6.88 | 23.27±14.18 | 17.48±15.37 | 12.77±13.79 | 0.276 |
| Vessel score | 0.86±1.07 | 0.07±0.26 | 1.43±1.08 | 0.95±1.05 | 0.71±1.14 | 0.386 |
| Pre-operative laboratory results | ||||||
| Hemoglobin, g/L | 135.5±16.2 | 133.2±17.5 | 137.9±14.8 | 133.8±15.3 | 137.1±17.2 | 0.426 |
| Creatinine, μmol/L | 96.3±27.8 | 91.0±22.3 | 101.6±32.0 | 100.6±30.3 | 92.3±25.2 | 0.265 |
| Sodium, mmol/L | 139.1 ±2.9 | 139.1±2.7 | 139.2±3.1 | 138.8±2.7 | 139.5±3.0 | 0.438 |
| Diabetic BSL | 7 (13%) | 3 (11%) | 4 (14%) | 3 (11%) | 4 (14%) | 0.761 |
| Intra-operative variables | ||||||
| Bypass time, m | 84 (71.0–99.0) | 75 (68.5–84.0) | 98.5 (84.3–114.8) | 96 (76.0–110.0) | 75 (65.0–89.5) | 0.007 |
| Cross-clamp time, m | 63 (54.5–79.5) | 61.5 (54.5–66.3) | 77 (52.8–100.5) | 68 (60.0–87.0) | 58 (46.5–67.0) | 0.013 |
AVR, aortic valve replacement; BSL, blood sugar level; CABG, coronary artery bypass grafting; IVS, interventricular septal thickness (end-diastolic); LVEF, left ventricular ejection fraction; LA, left atrium; LVEDD, left ventricular end-diastolic diameter; LVPW, left ventricular posterior wall thickness; POAF, post-operative atrial fibrillation.
Intraoperative characteristics (Table 3) including longer bypass time (median 96 mins, range: 76.0–110.0 mins vs. median 75 mins, range: 65.0–89.5 mins; P=0.007) and cross-clamp time (median 68 mins, range: 60.0–87.0 mins vs. median 58 mins, range: 46.5–67.0 mins; P=0.013) were also associated with POAF. Furthermore, POAF was significantly associated with increased hospital stay (10.3±4.3 vs. 8.2±4.2 days; P=0.005) and ICU stay (3.8±1.9 vs. 2.4±1.1 days; P<0.005).
EAT measurements
EAT measurements on CT (Table 4) that demonstrated a trend to significance but with P>0.05 were inferior IV groove (1.67±0.45 vs. 1.47±0.37 cm; P=0.06) and RCA-FP (6.08±1.59 vs. 5.41±2.16 cm2; P=0.08). The sum of these two parameters was significantly associated with POAF (7.75±1.70 vs. 6.87±2.27; P=0.05).
Table 4
| EAT segments (cm) | All patients (n=56) | AVR (n=28) | CABG or CABG + AVR (n=28) | POAF positive (n=27) | POAF negative (n=29) | P value |
|---|---|---|---|---|---|---|
| Apical four chamber view | ||||||
| Pericoronary fat tissue (right AV groove) | 1.61±0.35 | 1.59±0.35 | 1.64±0.34 | 1.67±0.29 | 1.56±0.39 | 0.25 |
| Pericoronary fat tissue (left AV groove) | 1.30±0.29 | 1.29±0.33 | 1.32±0.25 | 1.33±0.25 | 1.29±0.33 | 0.53 |
| LV apex | 0.27±0.11 | 0.29±0.12 | 0.25±0.09 | 0.27±0.12 | 0.28±0.10 | 0.68 |
| RV apex | 0.59±0.24 | 0.63±0.26 | 0.55±0.21 | 0.58±0.22 | 0.59±0.26 | 1.00 |
| Short axis view | ||||||
| RV anterior free wall, superior | 1.30±0.47 | 1.33±0.47 | 1.26±0.48 | 1.28±0.47 | 1.32±0.48 | 0.79 |
| RV anterior free wall, inferior | 1.51±0.61 | 1.56±0.71 | 1.46±0.51 | 1.49±0.48 | 1.53±0.73 | 0.9 |
| RV superior wall | 1.24±0.43 | 1.30±0.50 | 1.18±0.34 | 1.22±0.32 | 1.26±0.51 | 0.73 |
| RV diaphragmatic wall | 0.61±0.20 | 0.60±0.23 | 0.61±0.17 | 0.58±0.20 | 0.63±0.20 | 0.32 |
| Superior interventricular groove | 2.68 0.76 | 2.51±0.80 | 2.84±0.69 | 2.75±0.83 | 2.61±0.69 | 0.69 |
| Inferior interventricular groove | 1.56±0.42 | 1.50±0.33 | 1.63±0.49 | 1.67±0.45 | 1.47±0.37 | 0.06 |
| LV lateral wall | 0.80±0.24 | 0.84±0.26 | 0.76±0.22 | 0.82±0.26 | 0.78±0.23 | 0.5 |
| Epicardial fat thickness, total | 13.47±1.68 | 13.44±2.78 | 13.5±2.62 | 13.64±2.47 | 13.31±2.89 | 0.63 |
| Short-axis view at the level of the mid LA | ||||||
| LA to pulmonary artery | 0.23±0.15 | 0.24±0.13 | 0.22±0.16 | 0.21±0.07 | 0.26±0.19 | 0.86 |
| LA to esophagus | 0.19±0.08 | 0.20±0.09 | 0.17±0.06 | 0.17±0.07 | 0.21±0.08 | 0.06 |
| LA to aorta | 0.32±0.21 | 0.23±0.10 | 0.40±0.25 | 0.34±0.23 | 0.30±0.19 | 0.46 |
| EAT area at level of RCA origin | ||||||
| RCA-FP area (diastole, cm2) | 5.73±1.92 | 5.83±2.14 | 5.63±1.70 | 6.08±1.59 | 5.41±2.16 | 0.08 |
| Sum of RCA-FP and IIG | 7.29±2.05 | 7.32±2.22 | 7.26±1.90 | 7.75±1.70 | 6.87±2.27 | 0.05 |
AV, atrioventricular; AVR, aortic valve replacement; CABG, coronary artery bypass grafting; EAT, epicardial adipose tissue; IIG, inferior interventricular groove; LV, left ventricular; LA, left atrium; POAF, post-operative atrial fibrillation; RCA, right coronary artery; RCA-FP,: right coronary artery fat pocket; RV, right ventricular.
POAF prediction scoring system
A novel POAF risk prediction scoring system was created, outlined in Table 1. Multivariate logistic analysis (Table 5) revealed the scoring system using CHA2DS2-VASc score only was not statistically significant in predicting POAF (OR, 5.916; 95% CI: 0.876–39.949; P=0.068). However, the CHA2DS2-VASc score and LA diameter was strongly associated with POAF (OR, 3.792; 95% CI: 1.142–12.586; P=0.029). Adding EuroSCORE II improved the scoring system and was most powerfully associated with POAF, with each increase in score raising the risk of POAF by 4.2x (OR, 4.201; 95% CI: 1.162–15.180; P=0.029). Adding EAT to the components of the scoring system was still associated with POAF (OR, 3.752; 95% CI; 1.083–12.998; P=0.037) but did not improve upon the simpler scoring system using clinical and echocardiographic variables.
Table 5
| Variable | Odds ratio | 95% CI | P value |
|---|---|---|---|
| A: CHA2DS2-Vasc | 5.916 | 0.876–39.949 | 0.068 |
| B: CHA2DS2-Vasc + LA diameter | 3.792 | 1.142–12.586 | 0.029 |
| C: CHA2DS2-Vasc + LA diameter + EuroSCORE II | 4.201 | 1.162–15.180 | 0.029 |
| D: CHA2DS2-Vasc + LA diameter + EAT | 3.375 | 1.062–10.725 | 0.039 |
| E: CHA2DS2-Vasc + LA diameter + EuroSCORE II + EAT | 3.752 | 1.083–12.998 | 0.037 |
EAT, epicardial adipose tissue; LA, left atrium.
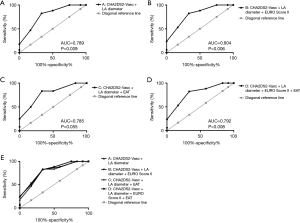
Receiver operator curve analysis (Figure 2) showed similar results with a score combining CHA2DS2-VASc score, LA diameter and EuroSCORE II having the highest association with POAF (AUC 0.804; P=0.006) (Figure 2B), but EAT did not improve the predictability (AUC 0.792; P=0.008) (Figure 2D). However, the combined graph showing the ROC curves (Figure 2E) revealed the models to have similar predictability and therefore warrants further investigations to determine the usefulness of EAT.
Discussion
Incidence of atrial arrhythmias after cardiac surgery has been reported to range from 10% to 65% (24,25). In our study, POAF was present in 48% of patients after cardiac surgery, including AVR alone or CABG with or without AVR. This study also confirmed that POAF most often occurs within the first few days after cardiac surgery, with the peak incidence on post-operative days two and three (26,27).
Increasing age has been correlated with POAF in previous studies. Age-related structural or electrophysiological changes appear to lower the threshold for postoperative atrial tachyarrhythmias in the elderly (1,7,26). Our study showed that higher age was associated with POAF. However, this could be confounded by higher average age of patients receiving CABG procedures (76±9 vs. 72±15 years), and POAF was more prevalent in patients undergoing CABG with or without AVR compared to AVR alone (64% vs. 32%, P=0.018).
Several mechanisms are involved in the pathogenesis of POAF. Dispersion in atrial refractoriness causes multiple local re-entry wavelets, which can be induced by surgical trauma, manipulation and myocardial ischemia. Furthermore, increased sympathetic activation, exaggerated inflammatory response and oxidative stress may contribute to the development of POAF (16,28). Related to this, cardiopulmonary bypass and cross-clamp times, type of cardioplegia, and CABG surgical techniques are regarded as critical in determining ischemic injury (29). Hence, this study explored POAF in patients undergoing AVR alone and patients undergoing on-pump CABG with or without AVR to compare the incidence of POAF and EAT characteristics in these two different populations. Patients undergoing AVR alone had shorter bypass time [75 (68.5–84.0) vs. 98.5 (84.3–114.8) mins] and cross-clamp time [61.5 (54.5–66.3) vs. 77 (52.8–100.5) mins] compared to patients undergoing CABG with or without AVR. CABG is a longer and more invasive procedure, which could contribute to the increased incidence of POAF compared to AVR alone (64% vs. 32%, P=0.018). Furthermore, this study demonstrated that bypass time (P=0.007) and cross-clamp time (P=0.013) are associated with POAF. A study has shown that while circulating levels of IL-18 protein was similar in patients undergoing CABG and valve replacement, their gene expression of IL-18 was higher in the EAT tissue of CABG patients compared to valve replacement patients (30). This adds to the notion that EAT has pro-inflammatory and pro-atherogenic properties (8-10) and could potentially contribute to increased POAF.
A number of pre-operative patient characteristics have been associated with POAF. The widely used CHA2DS2-VASc score that is used to stratify stroke risk in AF patients has also been shown to predict POAF (31,32). One study has found mean CHA2DS2-VASc scores in POAF and no-POAF groups to be 3.6±1.7 and 2.8±1.7 respectively (33). Our study also confirmed this finding with higher mean CHA2DS2-VASc scores in POAF patients (4.0±1.3 vs. 3.0±1.6).
Abnormally increased LV filling pressures gradually increase LA volumes (34). As a non-invasive marker of filling pressures, the E:E’ ratio is often used (35). Patients with POAF had numerically, but not significantly, higher E:E’ ratios (18.00±9.44 vs. 12.81±4.44; P=0.08). Patients with diastolic LV relaxation abnormalities are particularly prone to LA enlargement, and our study showed higher numerical values in the standard marker for diastolic dysfunction using echocardiography. The borderline P value is most likely due to our small sample size. A larger LA is associated with development of AF, and cardiovascular events (36,37). Our study showed that increased LA diameter has the potential to predict POAF in the setting of cardiac surgery. Echocardiography is routinely performed pre-operatively, and parameters such as LA diameter could be used in with other markers to predict POAF and screen for high-risk patients.
There is increasing interest in the role of EAT in predicting cardiovascular disease. The largest study stems from the Framingham Heart Study. 2,317 participants’ pericardial fat volume was characterized with CT, which predicted AF risk independent of other measures of adiposity (11). It is to be noted that as the Framingham Heart Study investigated multiple variables with multiple outcomes, there is a possibility of a type 1 statistical error. A recent study showed pericardial fat volume to be associated with POAF after CABG procedures (19). However, to the authors’ knowledge, no studies have used EAT to predict POAF after AVR surgery. EAT measurements in the clinical setting have been limited due to its time-consuming process, however automated approaches are currently being investigated in the literature (38,39). Saremi et al. have shown that diastolic RCA-FP surface area is a quick, reproducible estimate of total EAT (22). We have shown that a combination of an EAT thickness parameter (IIG) and an EAT area measurement (RCA-FP) is associated with POAF incidence after AVR alone or CABG with or without AVR. Using pre-operative contrast and non-contrast CT scans, these EAT parameters could potentially be measured routinely in the clinical setting to predict POAF risk. However from our data, such an approach does not appear to add further POAF predictive value compared to other POAF risk factors in the prediction scoring system, and its potential future role in pre-operative evaluation requires large-scale studies.
POAF is a complication that can have implications for our current ageing populations. Hospital length of stay after CABG can be extended by two to four days with additional healthcare costs due to POAF (27). Our study demonstrated that hospital and ICU stay was extended by two days. The burden of POAF could be prophylactically reduced by predicting high-risk patients using pre-operative characteristics identified in our POAF prediction scoring system. Assessed in a meta-analysis of ten trials, amiodarone is one such prophylaxis. Amiodarone was associated with a significant reduction in the rate of POAF or atrial flutter (40). However, all anti-arrhythmic drugs, including amiodarone, have significant potential side effects limiting their use routinely in all patients undergoing cardiac surgery. Targeting amiodarone therapy for those most likely to develop POAF is attractive, with potential savings in morbidity and duration of hospital stay.
In our study, multivariate logistic regression analysis and ROC analysis have shown that the POAF prediction scoring system using LA diameter, EuroSCORE II and CHA2DS2-VASc was strongly associated with POAF prior AVR alone or CABG with or without AVR (AUC 0.804; OR, 4.201; 95% CI: 1.162–15.180; P=0.029). Univariate analysis revealed the sum of IIG and RCA-FP to be associated with POAF (7.75±1.70 vs. 6.87±2.27; P=0.05), however the addition of EAT to the POAF prediction scoring system did not improve the risk prediction model for POAF (AUC 0.79; OR, 3.752; 95% CI: 1.083–12.998; P=0.037). The POAF prediction scoring system we have described in this study could help clinicians recognize high-risk patients for POAF preventative treatment before cardiac surgery.
There are several limitations to the study. Firstly, a major limitation of this study was the small sample size and therefore the results would be statistically underpowered. Further large-scale studies are required to validate the usefulness and accuracy of the POAF prediction score. Much of the data relied on the use of a database from a single centre, however our primary database was validated using demographic variables from two sources, independent of each other. Secondly, this was a retrospective study and as such there may be a lack of standardization in the data collected especially in regard to the CT data and pre-operative investigative data. Neither EuroSCORE II nor CHA2DS2-VASc is used conventionally to predict AF incidence. However, the increased mortality and morbidity risk that EuroSCORE II predicts may be related to longer stays in hospital due to complications including POAF. These variables are likely to be interrelated; therefore a combined scoring system such as the one outlined in this paper, could be useful. Furthermore, post-discharge POAF episodes were not included; therefore POAF incidence could be underestimated. However, as demonstrated in our study, POAF most likely occurs on post-operative days two and three. Patients with pacemakers and permanent AF were excluded, therefore the usefulness of EAT measurements for predicting POAF in these patients is still to be determined.
Conclusions
This study identifies a novel model to assess POAF risk using clinical pre-operative characteristics of patients undergoing AVR alone or CABG with or without AVR. The combination of EuroSCORE II, CHA2DS2-VASc and LA diameter was strongly associated with POAF. EAT did not add further strength in predicting POAF in our scoring system. Given the small sample size, any potential role for EAT in predicting POAF would need validation in large-scale studies.
Acknowledgments
The authors thank Professor Max Bulsara and Dr. Raoul Oehmen for their statistical expertise, and Mr. Nigel Bruer and Ms. Roberta Barbosa for their administrative assistance.
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jxym.2018.07.04). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Human Research Ethics Committee of The University of Notre Dame Australia and written informed consent was obtained from all patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Creswell LL, Schuessler RB, Rosenbloom M, et al. Hazards of postoperative atrial arrhythmias. Ann Thorac Surg 1993;56:539-49. [Crossref] [PubMed]
- Maisel WH, Rawn JD, Stevenson WG. Atrial fibrillation after cardiac surgery. Ann Intern Med 2001;135:1061-73. [Crossref] [PubMed]
- Tapio H, Jari H, Kimmo M, et al. Prevention of atrial fibrillation after cardiac surgery. Scand Cardiovasc J 2007;41:72-8. [Crossref] [PubMed]
- Lee JK, Klein GJ, Krahn AD, et al. Rate-control versus conversion strategy in postoperative atrial fibrillation: a prospective, randomized pilot study. Am Heart J 2000;140:871-7. [Crossref] [PubMed]
- LaPar DJ, Speir AM, Crosby IK, et al. Postoperative atrial fibrillation significantly increases mortality, hospital readmission, and hospital costs. Ann Thorac Surg 2014;98:527-33; discussion 33. [Crossref] [PubMed]
- Amar D, Shi W, Hogue CW Jr, et al. Clinical prediction rule for atrial fibrillation after coronary artery bypass grafting. J Am Coll Cardiol 2004;44:1248-53. [Crossref] [PubMed]
- Mathew JP, Fontes ML, Tudor IC, et al. A multicenter risk index for atrial fibrillation after cardiac surgery. JAMA 2004;291:1720-9. [Crossref] [PubMed]
- Iacobellis G, Corradi D, Sharma AM. Epicardial adipose tissue: anatomic, biomolecular and clinical relationships with the heart. Nat Clin Pract Cardiovasc Med 2005;2:536-43. [Crossref] [PubMed]
- Mazurek T, Zhang L, Zalewski A, et al. Human epicardial adipose tissue is a source of inflammatory mediators. Circulation 2003;108:2460-6. [Crossref] [PubMed]
- Ouwens DM, Sell H, Greulich S, et al. The role of epicardial and perivascular adipose tissue in the pathophysiology of cardiovascular disease. J Cell Mol Med 2010;14:2223-34. [Crossref] [PubMed]
- Thanassoulis G, Massaro JM, O'Donnell CJ, et al. Pericardial fat is associated with prevalent atrial fibrillation: the Framingham Heart Study. Circ Arrhythm Electrophysiol 2010;3:345-50. [Crossref] [PubMed]
- Ding J, Hsu FC, Harris TB, et al. The association of pericardial fat with incident coronary heart disease: the Multi-Ethnic Study of Atherosclerosis (MESA). Am J Clin Nutr 2009;90:499-504. [Crossref] [PubMed]
- Mahabadi AA, Massaro JM, Rosito GA, et al. Association of pericardial fat, intrathoracic fat, and visceral abdominal fat with cardiovascular disease burden: the Framingham Heart Study. Eur Heart J 2009;30:850-6. [Crossref] [PubMed]
- Ding J, Kritchevsky SB, Harris TB, et al. The association of pericardial fat with calcified coronary plaque. Obesity (Silver Spring) 2008;16:1914-9. [Crossref] [PubMed]
- Groves EM, Erande AS, Le C, et al. Comparison of epicardial adipose tissue volume and coronary artery disease severity in asymptomatic adults with versus without diabetes mellitus. Am J Cardiol 2014;114:686-91. [Crossref] [PubMed]
- Echahidi N, Pibarot P, O'Hara G, et al. Mechanisms, prevention, and treatment of atrial fibrillation after cardiac surgery. J Am Coll Cardiol 2008;51:793-801. [Crossref] [PubMed]
- Jakubova M, Mitro P, Stancak B, et al. The occurrence of postoperative atrial fibrillation according to different surgical settings in cardiac surgery patients. Interact Cardiovasc Thorac Surg 2012;15:1007-12. [Crossref] [PubMed]
- Hogue CW Jr, Hyder ML. Atrial fibrillation after cardiac operation: risks, mechanisms, and treatment. Ann Thorac Surg 2000;69:300-6. [Crossref] [PubMed]
- Drossos G, Koutsogiannidis CP, Ananiadou O, et al. Pericardial fat is strongly associated with atrial fibrillation after coronary artery bypass graft surgerydagger. Eur J Cardiothorac Surg 2014;46:1014-20; discussion 20. [Crossref] [PubMed]
- Bucher AM, Joseph Schoepf U, Krazinski AW, et al. Influence of technical parameters on epicardial fat volume quantification at cardiac CT. Eur J Radiol 2015;84:1062-7. [Crossref] [PubMed]
- Yorgun H, Canpolat U, Aytemir K, et al. Association of epicardial and peri-atrial adiposity with the presence and severity of non-valvular atrial fibrillation. Int J Cardiovasc Imaging 2015;31:649-57. [Crossref] [PubMed]
- Saremi F, Mekhail S, Sefidbakht S, et al. Quantification of epicardial adipose tissue: correlation of surface area and volume measurements. Acad Radiol 2011;18:977-83. [Crossref] [PubMed]
- European Heart Rhythm A. Guidelines for the management of atrial fibrillation: the Task Force for the Management of Atrial Fibrillation of the European Society of Cardiology (ESC). Europace 2010;12:1360-420. [Crossref] [PubMed]
- Mathew JP, Parks R, Savino JS, et al. Atrial fibrillation following coronary artery bypass graft surgery: predictors, outcomes, and resource utilization. MultiCenter Study of Perioperative Ischemia Research Group. JAMA 1996;276:300-6. [Crossref] [PubMed]
- van Dijk D, Nierich AP, Jansen EW, et al. Early outcome after off-pump versus on-pump coronary bypass surgery: results from a randomized study. Circulation 2001;104:1761-6. [Crossref] [PubMed]
- Zaman AG, Archbold RA, Helft G, et al. Atrial fibrillation after coronary artery bypass surgery: a model for preoperative risk stratification. Circulation 2000;101:1403-8. [Crossref] [PubMed]
- Aranki SF, Shaw DP, Adams DH, et al. Predictors of atrial fibrillation after coronary artery surgery. Current trends and impact on hospital resources. Circulation 1996;94:390-7. [Crossref] [PubMed]
- Maesen B, Nijs J, Maessen J, et al. Post-operative atrial fibrillation: a maze of mechanisms. Europace 2012;14:159-74. [Crossref] [PubMed]
- Peretto G, Durante A, Limite LR, et al. Postoperative arrhythmias after cardiac surgery: incidence, risk factors, and therapeutic management. Cardiol Res Pract 2014;2014:615987. [Crossref] [PubMed]
- Dozio E, Dogliotti G, Malavazos AE, et al. IL-18 level in patients undergoing coronary artery bypass grafting surgery or valve replacement: which link with epicardial fat depot? Int J Immunopathol Pharmacol 2012;25:1011-20. [Crossref] [PubMed]
- Chao TF, Liu CJ, Chen SJ, et al. CHADS2 score and risk of new-onset atrial fibrillation: a nationwide cohort study in Taiwan. Int J Cardiol 2013;168:1360-3. [Crossref] [PubMed]
- Chua SK, Shyu KG, Lu MJ, et al. Clinical utility of CHADS2 and CHA2DS2-VASc scoring systems for predicting postoperative atrial fibrillation after cardiac surgery. J Thorac Cardiovasc Surg 2013;146:919-26.e1. [Crossref] [PubMed]
- Kashani RG, Sareh S, Genovese B, et al. Predicting postoperative atrial fibrillation using CHADS-VASc scores. J Surg Res 2015;198:267-72. [Crossref] [PubMed]
- Dokainish H, Nguyen JS, Sengupta R, et al. Do additional echocardiographic variables increase the accuracy of E/e' for predicting left ventricular filling pressure in normal ejection fraction? An echocardiographic and invasive hemodynamic study. J Am Soc Echocardiogr 2010;23:156-61. [Crossref] [PubMed]
- Daneshvar D, Wei J, Tolstrup K, et al. Diastolic dysfunction: improved understanding using emerging imaging techniques. Am Heart J 2010;160:394-404. [Crossref] [PubMed]
- Kizer JR, Bella JN, Palmieri V, et al. Left atrial diameter as an independent predictor of first clinical cardiovascular events in middle-aged and elderly adults: the Strong Heart Study (SHS). Am Heart J 2006;151:412-8. [Crossref] [PubMed]
- Olshansky B, Heller EN, Mitchell LB, et al. Are transthoracic echocardiographic parameters associated with atrial fibrillation recurrence or stroke? Results from the Atrial Fibrillation Follow-Up Investigation of Rhythm Management (AFFIRM) study. J Am Coll Cardiol 2005;45:2026-33. [Crossref] [PubMed]
- Spearman JV, Meinel FG, Schoepf UJ, et al. Automated quantification of epicardial adipose tissue using CT angiography: evaluation of a prototype software. Eur Radiol 2014;24:519-26. [Crossref] [PubMed]
- Mihl C, Loeffen D, Versteylen MO, et al. Automated quantification of epicardial adipose tissue (EAT) in coronary CT angiography; comparison with manual assessment and correlation with coronary artery disease. J Cardiovasc Comput Tomogr 2014;8:215-21. [Crossref] [PubMed]
- Aasbo JD, Lawrence AT, Krishnan K, et al. Amiodarone prophylaxis reduces major cardiovascular morbidity and length of stay after cardiac surgery: a meta-analysis. Ann Intern Med 2005;143:327-36. [Crossref] [PubMed]
Cite this article as: Han MH, Playford D, vanden Driesen R, Judkins C. Predictors of post-operative atrial fibrillation in patients undergoing cardiac surgery: the potential role of epicardial adipose tissue. J Xiangya Med 2018;3:31.


