
The chimeric lateral femoral condyle flap for reconstruction of a long bone defect in the hand
Introduction
The medial femoral condyle flap was first described by Sakai et al. as a free vascularised bone graft based on the descending genicular artery (DGA) for treatment of fracture non-unions of the upper extremity (1). Through the instrumental contributions of Bakri et al., this flap became one of the work-horse flaps for treatment of recalcitrant bone non-unions or avascular necrosis of the wrist bones and in selected cases, also for functional soft tissue reconstruction (2-5). In a cadaver study, Laitung et al. described for the first time the possible harvesting of fasciocutaneous flaps from the distal lateral thigh based on perforators of the superolateral genicular artery (SLGA) (6). Later on, Wong et al. have shown a constant presence of the SLGA in 31 cadavers and its ability to sustain, on its deep branch, several types of bone flaps from the lateral femoral condyle (LFC) (7).
Recent studies from Parvizi et al. (3,4,8) brought further evidence on the anatomy and clinical importance of the SLGA and its capacity to sustain a wide variation of bone flaps (e.g., corticoperiosteal and corticocancelous) which can be also harvested with skin or fascial components as chimeric flaps. When comparing the diameter of the SLGA with its counterpart the DGA in 28 cadavers, Bürger et al. found it’s caliber to be constantly bigger than the latter (4). It’s length however, from its origin from the popliteal artery up to the point where it branches into the superficial and deep branch, was found to be shorter than of the DGA, one point that needs consideration especially when reconstructing defects where the recipient vessels are not in the immediate proximity of the defect (4).
However, besides Parvizi et al. that successfully transferred one composite SLGA flap including skin and part of the iliotibial band for functional reconstruction of the Achilles tendon (3), the clinical experience using this flap remains anecdotal. To complement their findings, here we describe the surgical anatomy of the SLGA and the use of a SLGA-based chimeric bone flap from the LFC to reconstruct a large defect of the fourth metacarpal after excision of a massive painful enchondroma.
Surgical anatomy of the SLGA
The SLGA arises from the popliteal artery and courses laterally up to the latero-dorsal margin of the distal femur shaft. From here, it turns ventrally dividing into the superficial patellar and deep articular branches. The deep branch lies in close contact with the periosteum of the LFC, giving two end-branches (transverse and distal) that supply the entire surface of the later, up to the cartilage of lateral margin of the knee joint. On its course, the SLGA provides several perforators supplying the lateral surface of the skin of the distal third of the thigh, up to the lateral margin of the patella ventrally (i.e., perforators based on the superficial patellar branch) and the lateral margin of the tibial head dorsally (i.e., perforators arising either from the main SLGA trunk or from the deep articular branch). These perforators branch from the SLGA then course either anterior, posterior or through the distal part of the iliotibial tract, to proceed towards the skin (Figure 1).
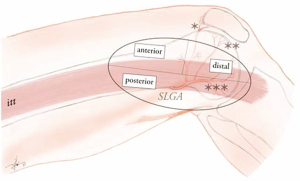
Considering the course of the SLGA and its constant anatomy, this provides the possibility to harvest a wide array of bone flaps from corticoperiosteal to corticocancelous constructs. However, the amount of corticocancelous bone which can be harvested without destabilising the LFC remains limited, which makes this type of flap ideally suited for reconstruction only of small to medium sized bones of the hand and wrist that require a vascularized bone graft. Complex bone defects concomitant with missing tendons or skin, can be reconstructed simultaneously if using chimeric flap designs, using appropriate SLGA branches that are able to support skin or fascial components from the distal iliotibial tract.
Due to its relative superficial location, the LFC can be approached either above or through the iliotibial tract. If the transverse SLGA branch will be harvested, an approach from above the iliotibial tract opens the ideal surgical field. The main disadvantage of this choice is that the flap pedicle will have to be kept short (2.5–3 cm) since the main trunk of the SLGA is poorly accessible for dissection. In addition, due to the small artery and vein caliber at this level, supra-microsurgery techniques might be mandatory for revascularisation. If a longer pedicle is needed, the approach through the iliotibial tract itself provides access to the full length of the SLGA (up to 5 cm) up to is emergence from the popliteal artery, and also to its distal articular branch.
Flap harvesting technique
The patient is placed supine on the operating table with a small pillow under the gluteal region on the side where the flap harvesting is planned. This enables a stable position of the flexed knee during the operation. To facilitate identification of vascular structures the flap is harvested without using an Esmarch bandage. We use a curvilinear incision on the lateral distal thigh. Depending on the tissue requirements, such if skin is needed, the necessary perforator can be identified using a hand-held audible doppler or doppler-ultrasound. To avoid wound break-down or painful scars, skin paddles should be designed parallel with the axis of the extremity and away from the lateral flexion skin fold of the knee.
Once the skin is incised, careful examination in a layer just above the iliotibial tract will localized the perforator arising from the SLGA system that has pierced the iliotibial. The main SLGA trunk is approached through the iliotibial tract, which is incised in its length for about 6 cm above the piercing point of the skin perforator. The distal-lateral part of the vastus laterals muscle is gently elevated off the LFC and held away using a Henley self-retractor. By following this maneuver, the transverse and distal branches of the SLGA should come into view.
Often the distal SLGA branch will be chosen to supply the bone graft. First, however, the SLGA is carefully elevated from the femoral shaft by ligation of several minor side-branches and freed on its entire length towards the popliteal artery. Access into the lateral popliteal fossa is achieved by careful separation of the SLGA from tight fascial reflections on the lateral margin of the distal femur shaft. Once the popliteal fossa is entered the dissection of the entire SLGA trunk is straight-forward and completed back to its popliteal origin.
Next, the boundaries of the desired bone graft are marked, and the corresponding periosteum is incised using monopolar cautery. Care before completing this cut is to make sure that several periosteal vessels distal to the bone harvesting border are first controlled by fine ligatures or bipolar cautery. This prevents troublesome bleeding later. After the periosteum has been scored, the bone graft is harvested using a fine oscillating saw and fine bone chisels. Once again great care must be taken to avoid fracture of the cancellous portion of the graft while extracting the bone from the LFC.
After the bone component has been freed, completion of dissection of the perforator and its skin island will be the last step in harvest of this as a chimeric flap Active spontaneous bleeding at the edges of the bone transplant should be noted at this time if circulation is indeed adequate. Once the recipient area has been prepared, the flap pedicle is ligated using titanium clips, directly above its emergence from the popliteal vessels, to make this a free flap.
Proper treatment of the donor site itself will avoid later difficulties. The bone defect so created in the LFC is filled with allogeneic demineralised bone chips, the iliotibial tract is closed over a drainage tube, and the skin is closed in anatomical layers. A compressive bandage of the entire lower extremity will minimize edema.
Case presentation
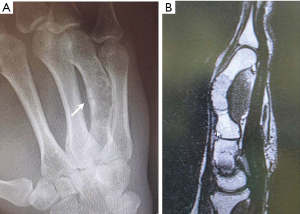
A 26-year-old patient was seen in consultation for a fast growing painful tumour of the right hand. Clinical exam showed a slight painful, fixed mass projecting into the skin of the fourth ray with no other functional deficits. CT examination revealed an advanced enchondroma infiltrating the entire shaft of the fourth metacarpal with a pathological fracture of the dorsal cortex (Figure 2). Due to the length of the bone involved, the location of the tumour and the presence of the pathological fracture, bone-sparing surgery with enchondroma enucleation and non-vascularised bone grafting was no longer the treatment option in our opinion.
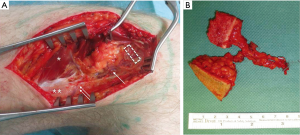
To accomplish our goal, complete excision of the affected metacarpal shaft and direct reconstruction using a free chimeric LFC flap with a perforator flap skin island as a monitor was performed. A two-team approach allowed simultaneous tumour excision and flap harvesting. A 4.5 cm × 1.5 cm × 0.6 cm (length × width × thickness) long corticocancelous bone was harvested from the LFC together with a 3.5 cm × 2 cm skin island based on one SLGA perforator (Figure 3A). The SLGA was dissected down to its origin into the popliteal artery, obtaining a pedicle length of 4.8 cm (Figure 3B).
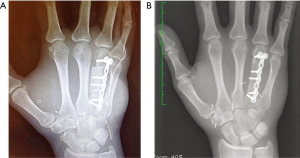
After tumour excision, the bone component was trimmed down to fit the bone defect, inserted, and stabilised with a titanium 2-mm locking plate and screws (Figure 4A). The flap pedicle was tunnelled under the skin and anastomosed end-to-side to the dorsal radial artery in the anatomical snuff box and end-to-end to a nearby superficial vein. The sentinel monitor perforator flap was sutured above the site of bone reconstruction allowing a tension-free closure. The donor site was closed per our previously outlined routine.
The hand was immobilised for 4 weeks in an intrinsic-plus forearm splint. Postoperative flap monitoring was according to our standard protocol for all free flaps which included hourly clinical observation (turgor, capillary pulse) and transcutaneous audible doppler examination (8 Mhz Doppler probe) over 5 days. During the same interval, to decrease the possible temperature-related postoperative vascular spasm issues, we use for all free flaps continuous warm air at 24 °C. Progressive active ambulation with assistance was allowed starting with postoperative day 1. The flap survived completely and the patient was fully ambulating with no assistance by the postoperative day 8.
After postoperative week 4, X-ray showed progressive callus formation at the bone reconstruction level. The cast was removed and the patient underwent a 2-month hand-therapy program including progressive joint mobilisation, lymph drainage and scar massage for the affected hand. Three month postoperatively, the function of the right hand was adequately restored allowing the patient to return to his full-time job as mechanic. Nine months postoperatively, an X-ray examination showed a complete integration and remodelling of the bone transplant (Figure 4B) with a normal functional hand (Figures 5,6). At the patient’s request, one year postoperatively, the skin island of the flap was resected and the wound closed primarily. The QuickDASH score performed 2 years postoperatively has shown (value =24.2) an acceptable level of disability.


Discussion
The search continues to find appropriate donor sites for vascularized bone grafts for hand defects. These will usually be relatively small and support corticoperiosteal or corticocancellous if not sometimes cartilages graft options that may be needed. The medial femoral condyle donor site has subsequently proven to be quite capable to meet these indications (1-5). However, if unavailable for whatever reason, it is always preferable to have yet another option. Its mirror image, the LFC as we and others have tried to demonstrate (7,8), represents a reliable donor area for vascularised bone flaps based on the superior lateral genicular artery. In addition, this choice can also allow the formation of chimeric flaps that include also fascial or skin components if desirable.
Versatility and reliability of the LFC does not yet have solid clinical evidence of its counterpart, the medial femoral condyle flap, the potential for pathological fractures, and the small size of these often <1 mm pedicle vessels should be concerns requiring utmost care when determining the amount of bone that must be harvested and the length of the pedicle needed to reach a potential recipient site. This case confirms that use of the LFC flap is possible, but further corroboration needs to be forthcoming.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Geoffrey Hallock, Juyu Tang) for the series “Perforator Flap” published in Journal of Xiangya Medicine. The article has undergone external peer review.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jxym.2018.04.06). The series “Perforator Flap” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sakai K, Doi K, Kawai S. Free vascularized thin corticoperiosteal graft. Plast Reconstr Surg 1991;87:290-8. [Crossref] [PubMed]
- Bakri K, Shin AY, Moran SL. The vascularized medial femoral corticoperiosteal flap for reconstruction of bony defects within the upper and lower extremities. Semin Plast Surg 2008;22:228-33. [Crossref] [PubMed]
- Parvizi D, Vasilyeva A, Wurzer P, et al. Anatomy of the Vascularized Lateral Femoral Condyle Flap. Plast Reconstr Surg 2016;137:1024e-32e. [Crossref] [PubMed]
- Bürger HK, Windhofer C, Gaggl AJ, et al. Vascularized medial femoral trochlea osteochondral flap reconstruction of advanced Kienböck disease. J Hand Surg Am 2014;39:1313-22. [Crossref] [PubMed]
- Jones DB Jr, Moran SL, Bishop AT, et al. Free vascularised medial femoral condyle bone transfer in the treatment of scaphoid nonuniouns. Plast Reconstr Surg 2010;125:1176-84. [Crossref] [PubMed]
- Laitung JK. The lower posterolateral thigh flap. Br J Plast Surg 1989;42:133-9. [Crossref] [PubMed]
- Wong VW, Bürger HK, Iorio ML, et al. Lateral femoral condyle flap: An alternative source of vascularized bone from the distal femur. J Hand Surg Am 2015;40:1972-80. [Crossref] [PubMed]
- Morsy M, Sur YJ, Saint-Cyr M, et al. Detailed anatomy of the superior genicular artery for design of a vascularised bone flap from the lateral femoral condyle. Plast Reconstr Surg 2015;136:14-5. [Crossref]
- Jiga LP, Jandali Z. Part 1: function of the right hand 2 years postoperatively. Asvide 2018;5:427. Available online: http://www.asvide.com/article/view/24432
- Jiga LP, Jandali Z. Part 2: function of the right hand 2 years postoperatively. Asvide 2018;5:428. Available online: http://www.asvide.com/article/view/24433
Cite this article as: Jiga LP, Jandali Z. The chimeric lateral femoral condyle flap for reconstruction of a long bone defect in the hand. J Xiangya Med 2018;3:18.


