
Anatomical basis of clavicular cross-area perforator flaps
Introduction
The skin around the clavicle has similar color and texture like that on the face and neck, with appropriate thickness. Thus, it is suitable for the preparation of pedicle transposition or free flaps, due to which, the head-neck and maxillofacial surgery have gained increasing attention in recent years. However, there was a great discrepancy in the nomination of the flap or its vascular morphology in some studies in China and worldwide. For example, at least six kinds of common nomination were noted: (I) supraclavicular axial patterned flap (1); (II) flap from the cervical cutaneous branch of transverse cervical artery (2); (III) supraclavicular neurovascular flap (3,4); (IV) supraclavicular fasciocutaneous island flap (5); (V) supraclavicular artery island flap (6,7); (VI) supraclavicular artery perforator flap (8-10). The analysis elaborated some common features as follows: (I) the flaps transverse the clavicle and contain a minimum of two perforasomes; especially, if the flaps are prefabricated, the volume of the flap carried by the distal perforator exceeds that of the anatomic donor area of the vessel pedicle (8,9); (II) the vessel pedicle of the flap is originated from the cervical cutaneous branch of the transverse cervical artery; (III) a majority of the investigators named the cervical cutaneous branch (superficial branch) of the transverse cervical artery as the supraclavicular artery.
Due to the limitations of the research conditions, methods, or clinical application, the above reports focused on different aspects. However, systematic studies of cutaneous (perforating) branches around the clavicle and their anastomosis have not yet been reported. In this study, 20 fresh cadaveric bodies were selected, and the microvascular identification and three-dimensional (3D) visualization integrated platform (11-16) was applied to observe the sources of cutaneous (perforating) branches around the clavicle and their anastomoses in all aspects-2D to 3D observation of general and digital anatomy .
Methods
A total of 20 fresh adult corpses were obtained from the Human Morphology Center of Medical College, University of South China, Hengyang, China and the Department of Anatomy, Wenzhou Medical University, Wenzhou China; 10 corpses from each institute. The corpses were subjected to whole-body lead oxide-gelatin mixture injection via unilateral femoral artery (11), followed by 64-slice spiral computed tomography (CT) scan of the layered anatomy of the specimen. The anatomical observation ranged from the upper chest and arm to the neck and shoulder. Simultaneously, the supraclavicular region was defined as the lower border that referred to the anterior margin of the clavicle and the upper border that referred to the connection between the acromion and midpoint of the posterior margin of the sternocleidomastoid muscle. On the other hand, the medial border referred to the connection between the midpoint of the posterior margin of the sternocleidomastoid muscle and the medial part of the origin of the sternocleidomastoid muscle.
Local 3D modeling
The scanned data were stored in the DICOM format and imported into a Mimics image workstation. Firstly, a fast-direct volume rendering (VR) was used for continuous 3D reconstruction from the superficial to the deeper region, which primarily revealed the locations of the major perforators in the neck, shoulder, and anterior chest wall, as well as their anastomosis. Subsequently, the other tools in the software such as Thresholding, Region growing, and Edit mask were used to construct the 3D visualization model, which was focused on the extraction and 3D reconstruction of the skeletons and arteries (13).
Positioning of perforators
The specimens were dissected in a layer-by-layer fashion to reveal the cutaneous nerves and their nutrient vessels, distinctly. Then, the cutaneous branches were carefully dissected and protected during which, we focused on anatomizing and observing the branches with an outer diameter ≥0.5 mm, including the site and the number of perforations. Radiography was applied while performing a layered anatomy until the source artery. Since single field radiography usually could not capture the whole corpse specimen, multiple X-ray images were essential. These X-ray data were input into the computer and the images processed, such as sorting, vitrification, calibration, and integration, using Photoshop (16).
Results
Distribution of cutaneous (perforating) branches around the clavicle
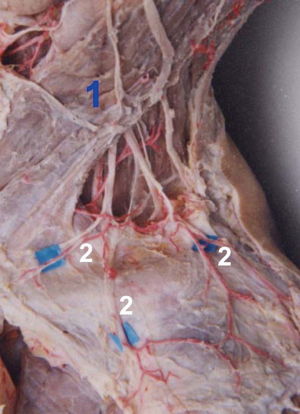
Abundant perforators in the above, below, and lateral (acromion) sides of the clavicle were observed. Four clusters of perforators were displayed in the 3D images: supraclavicular cluster, infraclavicular medial, middle and lateral clusters; these were anatomized in Figure 1. The 3D reconstruction was continuously performed from the superficial to the deeper layer, which showed that the four clusters of perforators originated from (I) transverse cervical artery (36/40, 90%) or suprascapular artery (4/40, 10%); (II) 2nd/3rd intercostal artery; (III) thoracoacromial artery; (IV) posterior circumflex humeral artery. The outer diameter of each cluster of perforators was listed in Table 1. The acromion branch was the largest lateral branch of the supraclavicular region, with a diameter of 1.2±0.4 mm at the origin. It was anatomized across the acromioclavicular joint to the ascending branch of the posterior circumflex humeral artery with a trunk length of 12.5±3.8 cm. In 8 specimens (20%), the acromion branches of the thoracoacromial artery (ab-TAA) traveled to the acromioclavicular joint via the anteroinferior margin of the clavicle, followed by anastomosis to the acromion branch of the transverse cervical artery (ab-TCA) and ascending branch of the posterior circumflex humeral artery, independently. In one case, the acromion branches of the suprascapular artery traveled upwards in the medial clavicle. Subsequently, it crossed the scapular gorges and then anatomized to the ab-TCA and ascending branch of posterior circumflex humeral artery separately. A total of 7 supraclavicular medial branches, originating from the sternocleidomastoid artery were thin, while one branch was relatively large with a diameter up to 0.8 mm.
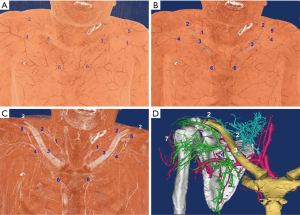
Table 1
| Source artery | Outer diameter (mm) |
|---|---|
| Posterior circumflex humeral artery | 0.8 |
| Thoracoacromial artery | 0.6 |
| Intercostal artery | 0.7 |
| Transverse cervical artery/suprascapular artery | 0.7 |
Vascular anastomosis of perforators
After 3D reconstruction, the cutaneous (perforating) branches around the clavicle, anatomized vessels among perforators, as well as subcutaneous vessel network, were observed distinctly. The three kinds of vascular anastomosis were observed: (I) anastomosis of acromion branch of the transverse cervical artery and ascending branch of the posterior circumflex humeral artery in Figure 1D and Figure 2; (II) anastomosis of the anterior thoracic branch of the transverse cervical artery and clavicular branch of the thoracoacromial artery; (III) anastomosis of sternocleidomastoid myocutaneous branch and ascending branch of the 2nd/3rd intercostal artery in Figure 1.
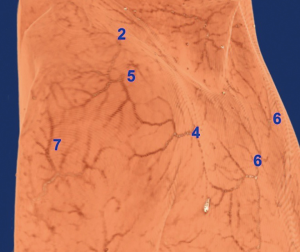
Nutrient vessels of supraclavicular nerve
The supraclavicular nerve was originated from the cervical plexus, which via the midpoint of the posterior margin of the sternocleidomastoid muscle, was gradually moved towards the inferolateral part in a fan-shape and divided into three branches: supraclavicular medial, middle nerves, and lateral nerves. These were distributed in the skin at the lateral neck, upper part of the anterior chest wall, and shoulder. In the supraclavicular part, the nutrient vessels of each nerve were originated from fasciocutaneous branches of the cervical transverse/suprascapular artery in Figure 3. Moreover, the nutrient vessels across the clavicle originated from the perforators in the corresponding area.
Discussion
The application of supraclavicular artery flap has an extended history. As early as 1949, Kazanjian and Converse reported the clinical application with a nomination of the acromial flap (17). In 1958, Kirschbaum used this flap in the treatment of severe post-burn sequel mentosternal contracture and achieved a satisfactory curative effect (17). In 1978, Mathes et al. reported the anatomy of the supraclavicular and shoulder flaps for the first time and use of the supraclavicular artery as the flap of the nutrient vessels for the repair of head and neck defects, which was termed as “the cervicohumeral flap” (18). However, the clinical application study by Blevins et al. (19) revealed that the distal necrosis rate of this flap was >20%, while Wilson [1978] achieved a success rate of up to 90% without delayed treatment (20), which led to the controversy about the feasibility of clinical application. Subsequently, Lamberty et al. (21) published that “the neck-humerus flap” should be a fascia flap instead of that previously mistaken for the (trapezius muscle) musculocutaneous perforator flap plus random flap (perforator plus flap). In China, Ma et al. (2) carried out the early studies in this area and reported the cervical cutaneous branches of the transverse cervical artery and their anastomosis to the cutaneous branches of the thoracoacromial artery and perforators of the internal thoracic artery. However, Pallua et al. continually investigated the clinical promotion and application of this flap since 1994 (8,9,22,23) with a success rate of 95%. The nomination of this flap was evolved from the supraclavicular island flap (SIF) in 1997 to the supraclavicular artery perforator (a-SAP) flap (8). Nevertheless, any detailed morphological studies have not yet been reported.
As a majority of the perforators in this region were originated from the transverse cervical artery with a relatively fixed position in the neck. These perforators and their vascular pedicles were primarily located within a 2-cm circle centered at a location 2 cm above the midpoint of the clavicle. Of these, the lateral perforators extended lateral-posteriorly, especially the acromion branch that was the largest and the longest. If this branch was considered as the vascular vessel for the procurement of the graft flap, the length of the pedicle was up to 13 cm according to the origin as the rotation point. According to the report by Pallua et al. (22), for a pedicle length of 20 cm, the flap could be easily rotated at 180° to repair the defects of the chest, neck, and cheek. The anterior perforators crossed the clavicle at the 1/3rd of the segment and extended anteroinferiorly; however, the sternoclavicular joint was subjugated by the upward extension of the ascending branches of the 2nd/3rd intercostal artery in Figure 1A,B. Since these branches were short, the graft flaps procured by considering the origin as the rotation point could only be transferred to the vicinity such as the anterior region of the neck or the submental region.
Their application areas exceeded the blood supply range of the supraclavicular artery, especially the volumes of the flaps carried in the distal perforasome that exceeded the anatomic donor range of the vascular pedicle after flap pre-preparation. The expanded flap reported by Yang et al. (24) displayed a procured area of 20 cm × 8 cm–23 cm × 16 cm). On the other hand, the island skin flap transferred by the tunnels, reported by Pallua et al. (22) revealed that the procured area was up to 16 cm × 32 cm); it could also be primarily sutured, resulting in good survival outcomes.
According to the hemodynamics, when the island flap was pedicled with the acromial branches of the transverse cervical artery, the distal ends of their axial blood vessels were only anatomized to the ascending branches of the posterior circumflex humeral artery under common circumstances. Concurrently, the blood supply method for the flaps is comprised of “the anatomic donor site and the dynamic donor site,” which benefits the survival of flaps. However, if the acromial branches of the thoracoacromial artery are inserted between them, the blood supply becomes “anatomic donor site + dynamic donor site + potential donor site,” which greatly increases the possibility of distal necrosis of the flaps. The results reported by Blevins et al. (19) might be associated with this issue. Nevertheless, if this flap was procured according to the method for procuring “fascia flap” (retaining deep fascia), the survival rate could be increased significantly (20,21).
Two types of cross-area anastomosis of anterior perforasomes occur in the supraclavicular region: (I) anastomosis of anterior thoracic branches of the transverse cervical artery and perforators of the thoracoacromial artery; (II) sternocleidomastoid myocutaneous perforators and ascending branches of the 2nd/3rd intercostal artery. These two types of perforators crossed the clavicle in different ways. In the case of the former, the anterior thoracic branches of the transverse cervical artery were moved downwards to cross the clavicle and anatomized with the perforators of thoracoacromial artery. For the latter, the ascending branches of the intercostal artery were extended upwards to cross the articulatio sternoclavicularis and anatomized with the sternocleidomastoid myocutaneous perforators in Figure 1.
Nomination of flaps: The perforator or cutaneous branch is defined as nutrient vessels supplying blood to the subcutaneous tissue and skin, which is emanated from the source vessel and crossed the deep fascia (25). It mainly includes two types: septocutaneous perforator and musculocutaneous perforator. Therefore, the supraclavicular superficial branches (cutaneous branches) emanated from the transverse cervical artery are both perforators, irrespective of acromion branches or anterior thoracic branches. Taken together, the supraclavicular artery mentioned in the previous literature is the perforator of the transverse cervical artery. To date, the flap in this region could not be termed as “supraclavicular perforator flap” (9) and is yet to be elucidated.
Since the axial blood vessels of flaps in this region are emanated from the cervical cutaneous branches of the transverse cervical artery, irrespective of acromion branches or anterior thoracic branches. Hence, they should be referred to as “cervical cutaneous branches or perforator flaps of transverse cervical artery” (2); however, according to the nomenclature principle of the flap, they can also be referred to as “supraclavicular (fascia) flap” according to the location (5,24,26). Nevertheless, several investigators named the cervical cutaneous branches (superficial branches) of the transverse cervical artery as the supraclavicular artery, and thus the supraclavicular artery flap was increasingly prevalent, as established based on usage.
Key points of applied anatomy: Preoperatively, a Doppler ultrasound was used for vascular exploration, which started from within a 2-cm circle centered at a location 2 cm above the midpoint of the clavicle to explore the supraclavicular and infraclavicular regions as well as the acromial region to identify the obvious perforating vessels.
In the acromial region, specific attention is focused on the infraclavicular lateral margin for exploring the presence of acromion branches of the thoracoacromial artery. If this branch of blood vessels extended directly to the acromioclavicular joint, it indicated that the flap pedicled with the acromion branch of a transverse cervical artery including three perforasomes in Figure 1D. In such cases, the flap should be dissected in the “fascia flap” manner (retaining deep fascia), or the distal end should be pre-dilated to increase the rate of survival (20,21,24). Otherwise, the possibility of distal flap necrosis would increase dramatically.
The anatomy of the vascular pedicle and its rotation point: The acromion branch of the transverse cervical artery is relatively long and is often considered as the axial blood vessel to procure the transposition flap, with the distal end up to the deltoid insertion. The key sites during the anatomy of the vascular pedicle are superior part of the acromioclavicular joint and the rotation point of the vascular pedicle. Since the superior part of the acromioclavicular joint is the junction of the perforator bodies with less subcutaneous fat, attention should be paid to the incision of the deep fascia to protect the integrity of the anastomotic vessels. Although the anterior chest branches of the transverse cervical artery are relatively short and the upgoing perforating branches of the thoracoacromial artery are not long, their conjoined flaps can be transposed to repair the anterior cervical and submental soft tissue defects. Irrespective of the acromion branch flaps of a transverse cervical artery or anterior thoracic branch flaps of the transverse cervical artery, vascular pedicle rotation point can be set at the beginning of the perforator while performing anterograde transposition. Thus, identifying the location is not difficult if the anatomy is based on the posterior margin of the sternocleidomastoid muscle, superior margin of the inferior belly of omohyoid muscle, and anterior margin of the trapezius muscle (occipital triangle) as markers. However, attention should be paid to avoid damaging the accessory nerve of the trapezius muscle during separation and ligation into the transverse cervical artery, as well as, the branches that locate in the deep trapezius muscle.
Conclusions
(I) Mimics can be used to conveniently and comprehensively analyze the cutaneous (perforating) branches and their vascular anastomosis. (II) Cross-area flaps can be procured according to the three types of anastomosis around the clavicle, which can be easily applied in the repair and reconstruction of maxillofacial and anterior cervical soft tissue defects. (III) In anterograde flap transposition, the key anatomical site of the vascular pedicle is located in the superior part of the acromioclavicular joint, and the vascular pedicle rotation point can be set in the occipital triangle.
Acknowledgments
Funding: This study was supported by the National Natural Science Foundation of China (81472104, 31371214).
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Geoffrey Hallock, Juyu Tang) for the series “Perforator Flap” published in Journal of Xiangya Medicine. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jxym.2018.04.01). The series “Perforator Flap” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement:
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Lamberty BG. The supra-clavicular axial patterned flap. Br J Plast Surg 1979;32:207-12. [Crossref] [PubMed]
- Ma XJ, Lu KH, Ai YF. Microsurgical anatomy of cervical transverse cervical branch flaps. Chinese Journal of Clinical Anatomy 1994;12:81-4.
- Chen HF, Liao JM, Xu CD, et al. Applied anatomy of supraclavicular neurocutaneous flaps. Chinese Journal of Clinical Anatomy 2003;21:204-6.
- Bo SP, Zhang SZ. Applied anatomy of supraclavicular neurocutaneous flaps. Chinese Journal of Practical Aesthetic and Plastic Surgery 2006;17:186-8.
- Chen WL, Zhang DM, Yang ZH, et al. Extended supraclavicular fasciocutaneous island flap based on the transverse cervical artery for head and neck reconstruction after cancer ablation. J Oral Maxillofac Surg 2010;68:2422-30. [Crossref] [PubMed]
- Chan JW, Wong C, Ward K, et al. Three- and four-dimensional computed tomographic angiography studies of the supraclavicular artery island flap. Plast Reconstr Surg 2010;125:525-31. [Crossref] [PubMed]
- Chen SS, Yao XW, Lu ZZ, et al. Clinical study of supraclavicular artery flap for repairing postoperative defects of maxillofacial tumor. Chinese Journal of Stomatological Research 2017;11:106-10. (Electronic Version).
- Pallua N, Wolter TP. Moving forwards: the anterior supraclavicular artery perforator (a-SAP) flap: a new pedicled or free perforator flap based on the anterior supraclavicular vessels. J Plast Reconstr Aesthet Surg 2013;66:489-96. [Crossref] [PubMed]
- Pallua N, Kim BS. Pre-expanded Supraclavicular Artery Perforator Flap. Clin Plast Surg 2017;44:49-63. [Crossref] [PubMed]
- Ismail H, Elshobaky A. Supraclavicular artery perforator flap in management of post-burn neck reconstruction: clinical experience. Ann Burns Fire Disasters 2016;29:209-14. [PubMed]
- Tang M, Geddes CR, Yang D, et al. Modified lead oxide-gelatin injection technique for vascular studies. Chin Clin Anat 2002;1:73-8.
- Zhang ZH, Li YB, Mei J, et al. Preliminary study of vascular 3D visualization using radiography. Chinese Journal of Clinical Anatomy 2006;24:255-8.
- Tang M, Yin Z, Morris SF. A Pilot Study on Three-Dimensional Visualization of Perforator Flaps by Using Angiography in Cadavers. Plast Reconstr Surg 2008;122:429-37. [Crossref] [PubMed]
- Mao YH, Ding MC, Zhou P, et al. Application of in vivo fluorescence staining combined with tissue transillumination in displaying skin microvascular structure. Acta Anatomica Sinica 2010;41:791-4.
- Tao Y, Hu S, Lui KW, et al. Quantitative regression analysis of the cutaneous vascular territories in a rat model. Surg Radiol Anat 2011;33:789-99. [Crossref] [PubMed]
- Mei J, Song TS, Dai KY, et al. Anatomical localization and quantitative study of human cutaneous arteries. Chinese Journal of Clinical Anatomy 2006;24:236-9.
- Kirschbaum S. Mentosternal contracture; preferred treatment by acromial (in charretera) flap. Plast Reconstr Surg Transplant Bull 1958;21:131-8. [Crossref] [PubMed]
- Mathes SJ, Vasconez LO. The cervicohumeral flap. Plast Reconstr Surg 1978;61:7-12. [Crossref] [PubMed]
- Blevins PK, Luce EA. Limitations of the cervicohumeral flap in head and neck reconstruction. Plast Reconstr Surg 1980;66:220-4. [Crossref] [PubMed]
- Wilson CA. The cervicohumeral flap. Plast Reconstr Surg 1978;62:288. [Crossref] [PubMed]
- Lamberty BG, Cormack GC. Misconceptions regarding the cervico-humeral flap. Br J Plast Surg 1983;36:60-3. [Crossref] [PubMed]
- Pallua N, Magnus Noah E. The tunneled supraclavicular island flap: an optimized technique for head and neck reconstruction. Plast Reconstr Surg 2000;105:842-51. [Crossref] [PubMed]
- Pallua N, Machens HG, Rennekampff O, et al. The fasciocutaneous supraclavicular artery island flap for releasing postburn mentosternal contractures. Plast Reconstr Surg 1997;99:1878-84. [Crossref] [PubMed]
- Yang Z, Liu W, Li YQ, et al. Dilated acromial supraclavicular flap for repair of cervical soft tissue defects. Chinese Journal of Aesthetic Medicine 2015;24:1-5.
- Zhang SM, Tang ML, Zhang WW, et al. Principles of nomencluture and clinical application of perforator flaps. Chinese Journal of Clinical Anatomy 2011;29:599-601.
- Alves HR, Ishida LC, Ishida LH, et al. A clinical experience of the supraclavicular flap used to reconstruct head and neck defects in late-stage cancer patients. J Plast Reconstr Aesthet Surg 2012;65:1350-6. [Crossref] [PubMed]
Cite this article as: Jiang Y, Zhou X, Kong F, Ding M, Mao Y, Cui H, Mei J, Tan J. Anatomical basis of clavicular cross-area perforator flaps. J Xiangya Med 2018;3:15.


