
Cardiac size and systolic function of HIV-infected Lagos children accessing routine care: a pilot study
Introduction
Sub-Saharan Africa is home to over 65% of the global paediatric HIV burden (1). As the HIV pandemic matures, more HIV-infected children are surviving into and beyond adolescence because of increasing access to highly active antiretroviral therapy (HAART). With this increasing survival, there is increasing reports of chronic diseases involving cardiopulmonary, neurologic, musculoskeletal and metabolic systems (2-4). Cardiovascular complications of HIV are one of the most commonly reported chronic complications of HIV (5-8).
HIV-infected children are at increased risk of chronic cardiac conditions owing to cumulative lifelong exposure of the myocardium to both the HIV virus, opportunistic pathogens and ARVs particularly during early developmental stages (7-10). Several mechanisms have been proposed for the deleterious effect of the virus on the heart, including direct viral invasion of the myocardium, chronic inflammation, endothelial dysfunction, autoimmunity, lung injury, malnutrition and drug-induced cardiotoxicity. Metabolic complications of prolonged ART such as dyslipidemias and insulin resistance have also been implicated (5,11).
A wide spectrum of echocardiographic abnormalities has been reported from studies in paediatric HIV-infected subjects, ranging from subclinical to florid pathology such as pericardial effusions (5,9,12-14). Other reported abnormal findings include left ventricular systolic and diastolic dysfunctions, and increased left ventricular mass (LVM)/left ventricular hypertrophy (LVH) (5,8,9,15,16). Echocardiographic abnormalities such as increased left ventricular dimensions, thickness and mass are associated with increased risk of fatal arrhythmias, sudden death, stroke, severe cardiac failure and myocardial infarction, independent of CD4 counts (14,17). Thus, routine baseline and serial cardiac evaluation of HIV-infected children with electrocardiographic and echocardiographic studies are advocated (5,7,17,18).
In Nigeria, studies on echocardiographic profile of HIV-infected children are relatively few (7,9,16,19,20). These studies reported prevalence rates of cardiovascular disorders ranging from 31–76%, based on clinical examination, electrocardiography and echocardiography. Specifically, no previous study has profiled the cardiovascular status of HIV-infected children in the current study center which attends to a high population of these subjects. Hence, the need for this study; this study aimed to document the prevalence and pattern of echocardiographic abnormalities of HIV-infected children assessing routine outpatient care at the paediatric HIV clinic of the Lagos State University Teaching Hospital in Lagos, South-West Nigeria. This pilot study will provide baseline data on the pattern of echocardiographic abnormalities in this population and establish the need for larger studies which will help guide local, and national, policy on routine cardiovascular screening and care.
Methods
The study was a prospective, cross-sectional study, carried out from 2011 to 2013 at the outpatient paediatric HIV clinic of LASUTH. The clinic has been running routine outpatient follow-up care of HIV-infected children since 2004 when it commenced as a pilot programme supported then by the US’ President’s Emergency Plan for AIDS Relief (PEPFAR). The clinic offers free comprehensive guidelines-based paediatric HIV care including HIV prevention, treatment, care and support services to enrolled HIV-infected children and their families. Baseline and follow-up haematological and biochemical investigations and ART are also provided free of charge.
Study population
This comprised of previously-diagnosed HIV-infected children aged 15 years and below accessing routine outpatient followed-up care in the unit and in whom, parents had given an informed consent. Patients with acute illnesses or those previously diagnosed with congenital or acquired heart diseases were excluded.
Data collection procedure
With the aid of a structured proforma, trained residents and interns obtained relevant socio-demographic and clinical information from participants whose parents or guardian consented in writing after explanation of the study. Eligible subjects were clinically evaluated for the presence of cyanosis, finger clubbing, tachypnea, tachycardia, cardiac murmur and tender hepatomegaly.
Subjects’ anthropometry (weight, height or length) were obtained following standard guidelines (21). Transcutaneous oxygen saturation (SpO2) was measured with a Nonin® finger pulse oximeter. The subjects also had their blood pressure measured in sitting position.
Clinical definitions
We defined significant tachycardia as heart rate greater than the upper limit of normal for age: greater than or equal to 160 beats/minute in infancy, 140 beats/minute at 2 years, 120 beats/min at 4 years, and 100 beats/minute at 6 years and above. Tachypnoea was defined as resting respiratory rate above or equal to 60 cycles per minute in the first two months of life, 50 cycles/minute or more from third to 12th month of life, 40 cycles and more beyond infancy. Congestive heart failure was defined as the presence of any two of tachycardia, tachypnoea or cardiomegaly WITH tender hepatomegaly (22). However, clinical presence of cardiomegaly determined by displaced apex beat was not assessed; LVH by echocardiography was thus employed as a criterion for cardiomegaly.
Echocardiography
Echocardiographic study was done for all subjects by a single well-experienced certified Paediatric Cardiologist in two-dimensional, Doppler and M-mode based on the American Society of Echocardiography (ASE) guidelines (23). The machine was a GE Vivid Q® 2-D echocardiography machine (reference number 14502 WP SN 2084) with facility for colour Doppler and M-mode. Measured parameters included main pulmonary artery (MPA) diameter, aortic root dimension (AO), left atrial dimension (LAD), left ventricular end diastolic diameter (LVEDD), left ventricular end systolic diameter (LVESD), interventricular septum (IVS) and left ventricular posterior wall diameter (LVPW). These parameters were used to derive the left ventricular ejection fraction (LVEF), left ventricular fractional shortening (LVFS), LVM and relative wall thickness (RWT) (23,24):
In order to correct for confounding effect of varying body size with age, the LVM was indexed to height raised to the power of 2.7 (height2.7) and a cut-off value of 51 g/m2.7 was used as indicative of increased LVM (25). LVEDD was expressed as z-scores based on available references and LVEDD z-score values >2 was defined as LV dilatation (26,27). LV systolic dysfunction was defined as LVSF <28% and dilated cardiomyopathy as LVSF <28% with LVD (28). Increased RWT was defined as RWT ≥0.42. RWT was used to classify subjects with increased LVM (LVH) as either concentric LVH (when both RWT and LVM are elevated) or eccentric LVH (when LVM is elevated with a normal RWT) (29).
Data of control subjects were extracted from an electronic database of the Paediatric Cardiology Unit. Control subjects were gender- and age-matched apparently normal (clinically stable HIV-uninfected subjects with structurally normal heart) who had their echocardiographic parameters documented as above by the same Paediatric Cardiologist.
Statistical analysis
Statistical analysis was performed using SPSS version 20 (IBM, Chicago, USA). Continuous variables were presented as mean (SD) or median (interquartile range) while categorical variables were presented as frequencies (%). Student t-test, or Mann-Whitney U-test, was used to compare continuous variables between HIV-infected subjects and controls. Chi square test, or Fisher exact test where appropriate, was used to compare proportions between the two groups. Level of statistical significance was set at P<0.05.
Results
The study cohort consisted of 41 HIV-infected children, with 41 age- and sex-matched controls. Two HIV-infected subjects were excluded from further analysis due to gross data entry errors; further analysis was thus based on 39 HIV-infected subjects with 41 control subjects.
Demographic characteristics of subjects and controls
Table 1 shows comparison of the demographic characteristics of the study and control groups; there was no significant difference in their baseline characteristics. The ages of the HIV-infected group ranged from 2.7 to 14 years while it ranged from 2.0 to 14 years among the controls. Fifty-four percent (54%) of the HIV-infected subjects were females. About half of the HIV-infected subjects were in the age group 5 to 10 years. The median ages (± interquartile range) of the study subjects and controls were 7.0±5.0 and 6.0±7.0 years respectively (P=0.585). All, but one, (97%) of the subjects were on HAART with 11(28%) of them on zidovudine-based regimen.
Table 1
| Demographic parameter | Group, N [%] | Total (N=80) [%] | P value | |
|---|---|---|---|---|
| Cases (N=39) | Controls (N=41) | |||
| Age (years)* | 7.0 (5.0) | 6.0 (7.0) | 0.585 | |
| Age group | ||||
| ≤5 | 12 [31] | 19 [46] | 31 [39] | |
| >5–10 | 19 [49] | 10 [24] | 29 [36] | |
| >10 | 8 [20] | 12 [30] | 20 [25] | |
| Total | 39 [100] | 41 [100] | 80 [100] | |
| Gender | ||||
| Male | 18 [46] | 19 [46] | 37 [46] | |
| Female | 21 [54] | 22 [54] | 43 [54] | |
| Total | 39 [100] | 41 [100] | 80 [100] | |
*, median with interquartile range (IQR); Mann-Whitney U-test.
Clinical findings in HIV-infected subjects
Table 2 shows anthropometric and clinical date of the HIV-infected subjects while Table 3 shows the frequency of clinical signs elicited during clinical examination. Of the 39 HIV-infected subjects, 8 (21%) had one or more clinical symptoms or signs. However, none met the criteria for congestive heart failure; tachypnoea, tachycardia or tender hepatomegaly occurred in isolation. The subject that had a cardiac murmur had no feature of heart failure and subsequent echocardiography did not reveal any cardiovascular abnormality.
Table 2
| Variable | N | Mean [SD] | Range |
|---|---|---|---|
| Weight (kg) | 38 | 22.3 [8.2] | 10.0–46.0 |
| Height (cm) | 37 | 117.3 [25.3] | 72.0–150.0 |
| Respiratory rate (c/min) | 38 | 28 [6] | 20–56 |
| SBP (mmHg) | 27 | 90 [11] | 70–110 |
| DBP (mmHg) | 27 | 56 [8] | 40–70 |
| SpO2 (%) | 22 | 98.4 [1.9] | 94–100 |
SBP, systolic blood pressure; DBP, diastolic blood pressure; SpO2, transcutaneous oxygen saturation; c/min, cycles per minute.
Table 3
| Clinical signs | Frequency |
|---|---|
| Dyspnea | 1 |
| Cyanosis | 0 |
| Tachypnea | 2 |
| Tachycardia | 5 |
| Cardiac murmur | 1 |
| Gallop rhythm | 0 |
| Tender hepatomegaly | 2 |
| Total | 11* |
*, some subjects had more than one clinical features.
Echocardiographic measurements of subjects and controls
Table 4 shows a comparison of the echocardiographic parameters of the HIV-infected subjects and matched control group. Although all the echocardiographic data (except RVAW and LVPWD) were larger in the HIV-infected group, only IVSD, LA and AO were significantly larger; that is HIV-infected group had significantly larger interventricular septal diameter (IVSD), LA and aortic diameter. The functional parameters (LVFS and LVEF) were not significantly different between the groups.
Table 4
| Echocardiographic measurements | Groups | t value | P value | |
|---|---|---|---|---|
| Cases | Control | |||
| MPA (cm) | 2.00±0.4 | 1.99±0.47 | 0.134 | 0.893 |
| RVAW (cm) | 0.39±0.10 | 0.39±0.12 | −0.216 | 0.829 |
| RVD (cm) | 1.20±0.38 | 1.09±0.43 | 1.191 | 0.237 |
| IVSD (cm) | 0.70±0.17 | 0.59±0.22 | 2.419 | 0.018* |
| LVEDD (cm) | 3.74±0.62 | 3.49±0.54 | 1.913 | 0.059 |
| LVPWD (cm) | 0.65±0.16 | 0.67±0.16 | −0.471 | 0.639 |
| LVEDS (cm) | 2.38±0.40 | 2.32±0.41 | 0.697 | 0.488 |
| AO (cm) | 2.14±0.30 | 1.95±0.42 | 2.329 | 0.023* |
| LAD (cm) | 2.56±0.41 | 2.25±0.50 | 3.034 | 0.003* |
| LAD/AO | 1.21±0.20 | 1.16±0.17 | 1.049 | 0.297 |
| LVFS (%) | 36.70±10.33 | 34.03±7.09 | 1.344 | 0.183 |
| LVEF (%) | 71.82±11.08 | 70.30±10.09 | 0.637 | 0.526 |
| LVM (g) | 61.61±32.68 | 48.49±30.90 | 1.854 | 0.023** |
| RWT | 0.36±0.09 | 0.39±0.09 | −1.722 | 0.089 |
*, P<0.05; **, Mann-Whitney U test. LV, left ventricle; MPA, main pulmonary artery; RVAW, right ventricular anterior wall; RVD, right ventricular dilatation; IVSD, interventricular septal diameter; LVEDD, left ventricular end diastolic diameter; LVPWD, left ventricular posterior wall in diastole; LVEDS, left ventricular end systolic diameter; AO, aortic root dimension; LAD, left atrial dimension; LVFS, left ventricular fractional shortening; LVEF, left ventricular ejection fraction; LVM, left ventricular mass, RWT, relative wall thickness.
Prevalence and pattern of cardiac abnormalities in HIV-infected subjects
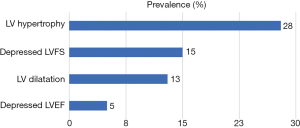
Eighteen (44%) subjects had abnormal echocardiographic abnormalities thus: 2 (5%), 5 (13%), 6 (15%) and 11 (28%) subjects, respectively, had depressed LVEF, LV dilatation, systolic dysfunction (depressed LVFS) and LVH (Figure 1). Four of those with LVH had concentric LVH while seven had eccentric LVH. None of the subjects fulfilled criteria for pericardial effusion or dilated cardiomyopathy. Four subjects had both LV dilatation and LVH. Only one of the subjects with LVH had elevated blood pressure (stage 1 hypertension) and the LVH type was eccentric. Of the 18 subjects with echocardiographic abnormalities, only 1 (0.1%) was symptomatic: a 42-month old boy with fever, mild tachypnoea and tender hepatomegaly but no tachycardia who had both LV dilatation and LVH.
Clinical characteristics of HIV-infected subjects with cardiac abnormalities
Subjects with cardiac abnormalities were younger, lighter and shorter than those with normal cardiac measurements and they also had lower systolic and diastolic blood pressure; albeit these differences were not statistically significant (Table 5).
Table 5
| Variable | Echocardiographic abnormalities | P value* | |
|---|---|---|---|
| Present, mean [SD] | Absent, mean [SD] | ||
| Age (years) | 6.4 [3.4] | 8.3 [2.7] | 0.052 |
| Weight (kg) | 20.1 [8.9] | 24.1 [7.2] | 0.131 |
| Length (cm) | 107.1 [19.4] | 120.1 [28.4] | 0.119 |
| SBP (mmHg) | 88 [11.9] | 91 [11.3] | 0.509 |
| DBP (mmHg) | 51 [12] | 57 [9.2] | 0.997 |
| SpO2 (%) | 99 [1] | 98 [2] | 0.210 |
*, independent t-test. SBP, systolic blood pressure; DBP, diastolic blood pressure; SpO2, transcutaneous oxygen saturation; SD, standard deviation.
A comparison of the distribution of subjects by age group between those with abnormal echocardiographic abnormalities and those with normal parameters shows that majority (56%) of those with abnormalities were less than 5 years of age; thus 83% of children less than 5 years had abnormal cardiac abnormalities (P=0.001; Table 6).
Table 6
| Age group | Echocardiographic abnormalities, n [%] | Total, n [%] | |
|---|---|---|---|
| Present | Absent | ||
| <5 years | 10 [83] | 2 [17] | 12 [100] |
| 5–10 years | 5 [26] | 14 [74] | 19 [100] |
| >10 years | 3 [38] | 5 [62] | 8 [100] |
| Total | 18 [46] | 21 [54] | 39 [100] |
Fisher Exact test, P=0.001.
Table 7 shows the distribution of each of LVFS and LVMI in the HIV-infected subjects based on age group. There was no significant difference in the distribution of abnormal LVFS among the age groups. However, most (73%) of the subjects with elevated LVMI (LVH) were less than five years of age.
Table 7
| Age groups | LVFS* (n) | LVMI** (n) | |||||
|---|---|---|---|---|---|---|---|
| Depressed | Normal | Total | Elevated | Normal | Total | ||
| <5 years | 2 | 9 | 11 | 8 | 2 | 10 | |
| 5–10 years | 2 | 16 | 18 | 2 | 16 | 18 | |
| >10 years | 2 | 6 | 8 | 1 | 7 | 8 | |
| Total | 6 | 31 | 37 | 11 | 25 | 36 | |
*, χ=0.831; **, Fisher Exact test, P=0.001. LVFS, left ventricular fractional shortening; LVEF, left ventricular ejection fraction.
Relationship between zidovudine exposure and cardiac abnormalities
Of the 36 subjects with data on ARV regimen, 25 subjects were on zidovudine-based regimen (Table 8). There was no significant difference between the prevalence of LVH between those on zidovudine-based regimen compared to those unexposed to zidovudine (P=0.703).
Table 8
| LVMI | Zidovudine exposure | Total | |
|---|---|---|---|
| Yes | No | ||
| Elevated | 7 | 4 | 11 |
| Normal | 18 | 7 | 25 |
| Total | 25 | 11 | 36 |
Fisher Exact test; P=0.703; LVMI, left ventricular mass index.
Discussion
We present data on echocardiographic characteristics of HIV-infected children on routine outpatient care at the paediatric HIV clinic of the Lagos State University Teaching Hospital, Lagos in South-West Nigeria. Majority of the study subjects were less than 10 years old, most presumed to have been infected via vertical transmission. Vertical transmission is still the predominant mode of transmission of HIV in paediatric population in the developing world due mainly to low coverage of PMTCT services (9,30).
The present study shows high prevalence of subclinical abnormal echocardiographic profile of HIV-infected children: almost half (46%) of the subjects had one or more abnormalities of cardiac size and/or function. This prevalence was higher than values of 7%, 27%, 37% in a Ugandan study and two separate Indian studies, respectively (30). A study conducted on Nigerian children in Lagos by Okoromah et al. (7) reported a higher prevalence of 76% based on abnormal clinical, electrographic and echocardiographic findings unlike the index study based only on abnormal echocardiographic parameters. Moreover, the subjects in the index study were stable HIV-infected children on outpatient care and were less ill compared to Okoromah study, which included acutely ill, hospitalized children. Another explanation for the widely disparate prevalence values from one study to another is the varying spectrum of echocardiographic abnormalities studied. For example, while the index study evaluated left ventricular systolic function, the Cameroonian study evaluated both diastolic and systolic functions of the right and left ventricles.
The subjects with cardiac abnormalities in the index study were largely asymptomatic as shown by the fact that one subject had clinical symptoms. This is similar to most reports in the post-HAART era as against the pre-HAART dominated by high prevalence of symptomatic cardiac disorders (7,30-34). In a study of Thai children by Pongprot et al. (35), almost all the 27 subjects studied presented with symptoms such as dyspnoea, oedema, finger clubbing, cyanosis and gallop rhythm. The index study was a prospective study of ambulatory outpatient subjects on ART in contrast to this Thai study, which was a retrospective study of ART-naïve children evaluated primarily for HIV-related cardiac disorders. More recent longitudinal studies have shown long-term ART to be cardio-protective at least in the first decade of life (6). The finding of low prevalence of cardiorespiratory symptoms in HIV-infected children with cardiac abnormalities may suggest that clinical symptoms and signs have low sensitivity in the early detection of HIV-associated cardiac dysfunctions. Hence, routine serial echocardiographic studies are mandatory in the early detection HIV-associated heart diseases.
Similar to findings by Okoromah et al. (7) most of the echocardiographic parameters were similar between HIV-infected children and controls.
Left ventricular dilatation (defined as LVEDD >2 z-score), a subclinical precursor to more life-threatening cardiac complications such as dilated cardiomyopathy and congestive heart failure, has been widely reported in HIV-infected subjects (7,8,13,32,34-37). The LVEDD of the study subjects were not significantly larger than those of controls. However, the P value was close to statistical significance. The index study observed a prevalence of LV dilatation of 12.8%; higher than prevalence of 1% and 7% in Cameroonian and Zimbabwean children respectively but lower than 37% reported in a European study (38). None of the subjects in the index study met criteria for dilated cardiomyopathy similar to a study of Cameroonian children (13). Anaemia has been reported to contribute to chamber dilatation in HIV-infected children (37). Recent reports suggest that the prevalence of dilated cardiomyopathy has remarkably declined since the advent and wide availability of HAART (7,13,19,28,34-37).
The right ventricular dimension of HIV-infected subjects was larger than that of controls, albeit non-significantly, in the present study. However, the prevalence of right ventricular dilatation (RVD) was not specifically determined. In a study of 100 Cameroonian children, Chelo et al. (13) reported a very high prevalence 76% whereas the prevalence of LV dilatation was just 1%. Surprisingly only 8% of the subjects with RVD had pulmonary hypertension presumably secondary to chronic HIV-associated lung disease. The authors suggested that the use of Caucasian references may have resulted in overdiagnosis of RVD in the cohort. Lower prevalence rates 14-30% have been reported in other African studies of HIV-infected children (17,32).
The present study observed a significantly larger LVM among subjects compared to controls. This is similar to findings in reports from other centres (7,9,20). The prevalence of LVH in this cohort (28%) was similar to 21% and 29% reported in studies of Nigerian (9) and Indian (31) children, respectively. It was lower than 67% in Zimbabwean (17) children but higher than 14% reported in another cohort of Nigerian children in northern Nigeria (9). Differences in the diagnostic criteria for LVH may partly contribute to the variations in reported prevalence, in addition to true geographic variations in disease pattern and distribution. For example, while the index study used LVM indexed to height raised to the power of 2.7 (ht2.7), a previous Nigerian study indexed LVM to body surface area (9) while another used unindexed LVM (7). The need to use indexed LVM, rather than LVM, arose because of the increasing cardiac size that normally occurs with increasing age and body composition in the paediatric age group (23). LVM indexed to ht2.7 has been reported to account better for the confounding influence of body fat on LVM compared to LVM indexed to BSA (7-9). Due to absence of local reference values for LVMI indexed to ht2.7, most studies, including the index study, have used the adult cut-off value of 51 g/m2.7 which has been shown to be imprecise in children because of its significant variation with height particularly in preadolescents. Use of percentile curves or z-scores has thus been proposed as better alternatives in children (39). However, absence of local references precludes use of these methods in the index study.
LVH and LVM are important parameters useful in prediction of risk of mortality and adverse cardiovascular events in HIV-infected children (40). Concentric LVH has been associated with increased cardiovascular morbidity and mortality in adults while those with eccentric LVH have an intermediated risk (41). Majority of the subjects with LVH in the present study were below five years of age. Other studies have also suggested this age group may be at higher risk for HIV-associated cardiac abnormalities (9,38). The reasons for this observation is not immediately apparent but may not be unconnected with the effect of long-term HAART: the longer the duration on HAART the smaller the incidence of cardiovascular complications of HIV (28).
LVH in HIV-infected subjects has been attributed to a subclinical myocardial inflammation, which underlies a spectrum of heart disease ranging from asymptomatic LV dilatation to full-blown dilated cardiomyopathy in later years. Hence, such individuals require long-term monitoring and, when necessary, interventions such as selenium supplementation and use of immunoglobulins which have been shown to reverse these cardiac aberrations (28,42).
Despite larger left atrial and ventricular sizes, the HIV-infected group had better left ventricular systolic function in the index study. However, the differences were not significant. Other authors reported significantly lower LVFS and LVEF in HIV-infected children compared to normal population (7,20,37). LVFS is a product of several cardiovascular processes such as preload, afterload, heart rate and cardiac contractility; derangement of any of these processes may thus result in abnormal LVFS (23,40). Singly or in conjunction with LVH, LVFS is a long-term predictor of mortality in HIV-infected children (18,40).
The prevalence of systolic dysfunction (defined as LVFS <28%) in the present study was lower than 35% reported by Okoromah et al. (7) a study conducted about seven to nine years earlier than the index study. Whereas the present study defined LV dysfunction as LVFS <28% the Okoromah study used a cut-off value of ≤25%. However, another study of Nigerian children by Ige et al. (19) reported a prevalence rate of 50% despite use of cut-off value of <28%. Another explanation of this disparity could be the fact that 97% of the subjects in the present study were on ART compared to 56% in the latter study; HIV-infected children on HAART have lower prevalence of LV dysfunction (37). A very low prevalence of
The presence of thicker ventricular wall, ventricular dilatation and LVH in ARV-exposed HIV-infected children, as observed in the index study, are postulated to be compensatory mechanisms that arise in response to HIV-associated anaemia and myocardial inflammation aimed at preserving cardiac output. However, as these responses progress a point is reached when they become detrimental resulting in reducing LVFS (37). Early detection of subclinical HIV-associated cardiovascular disorders is thus important to reduce the frequency of full-blown cardiovascular events. Treatment with drugs such as angiotensin converting enzyme inhibitors (ACEIs), intravenous immunoglobulins, selenium supplementation and beta-blockers like carvedilol may slow or reverse this disease progression (28-31).
Data on clinical and laboratory measures of disease severity were not available for the subjects in the index study. Hence, their association with cardiac abnormalities could not be assessed. Although cardiac abnormalities tend to be prevalent with declining immune status, CD4 count has been reported to be a poor marker of cardiovascular disorders (31,43).
Although drugs such as zidovudine and abacavir have been reported to have adverse cardiovascular effect when HIV-infected children are perinatally exposed to them, the LVMI of subjects on zidovudine-based therapy in the present study was not significantly different from those of subjects on other regimen (7,28). This is similar to other reports (17). Recent data from longitudinal studies strongly suggest that HAART is generally cardio-protective at least for the first decade of life after which this protective effect seem to decline gradually into adulthood (6,28,44).
The strengths of this study include use of a prospective design and exclusion of acutely ill patients. However, the small sample size limits generalization of the findings of the study to the larger population of HIV-infected children in our center or beyond. Nonetheless, the findings were not widely different from findings from other Nigerian studies with larger sample sizes (7,9,19,20). Use of control subjects obtained from our echocardiographic database is also a limitation as rigorous inclusion and exclusion criteria could not be applied to the control group. However, careful matching was done and the same cardiologist did the echocardiography for both groups, thus minimizing inter-rater bias. Also, this study did not evaluate the diastolic function of the subjects which may precede systolic dysfunctions in the spectrum of cardiac disease in HIV-infected children (45).
In conclusion, this study has highlighted the presence of asymptomatic borderline cardiac dysfunction in HIV-infected children and thus lends it voice to advocacy for the inclusion of baseline and follow-up echocardiography in the routine care of HIV-infected children (7,9,20,28). Scale-up of PMTCT services with wider access to early infant diagnosis will reduce the burden of vertically transmitted HIV infection and, by extension, its cardiac complications. There is also need for local reference values for paediatric echocardiographic parameters using current standards such as z-scores to enhance accurate diagnosis of childhood cardiac dysfunctions.
As HIV-infected children and adolescents with subclinical cardiac abnormalities grow into adulthood and get exposed to other cardiovascular risk factors such as hypertension, smoking, obesity or hyperlipidaemia there is concern that they may eventually become symptomatic (40). Large prospective longitudinal, preferably multi-center, studies, specifically in sub-Saharan African context, are thus needed to ascertain the current cardiac profile of HIV-infected children and elucidate it clinical determinants such as genetic factors and micronutrient deficiencies such as selenium deficiency. Lastly, where resources are scarce, perinatally-infected HIV-infected under-five children may be prioritized for detailed cardiovascular assessment.
Acknowledgments
The parents and patients who participated in this study are gratefully acknowledged.
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jxym.2018.03.04). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by Lagos State University Teaching Hospital (LREC.06/10/823). Informed consent was taken from all subjects.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- UNAIDS. UNAIDS Data 2017. Geneva, Switzerland, 2017.
- Vreeman RC, Scanlon ML, McHenry MS, et al. The physical and psychological effects of HIV infection and its treatment on perinatally HIV-infected children. J Int AIDS Soc 2015;18:20258. [Crossref] [PubMed]
- Sutcliffe CG, van Dijk JH, Bolton C, et al. Effectiveness of antiretroviral therapy among HIV-infected children in sub-Saharan Africa. Lancet Infect Dis 2008;8:477-89. [Crossref] [PubMed]
- Hazra R, Siberry GK, Mofenson LM. Growing up with HIV: children, adolescents, and young adults with perinatally acquired HIV infection. Annu Rev Med 2010;61:169-85. [Crossref] [PubMed]
- Gary W. AIDS and Other Manifestations of HIV Infection. Fourth. New York: Elsevier Academic Press, 2004:1000.
- Lipshultz SE, Williams PL, Wilkinson JD, et al. Cardiac status of children infected with human immunodeficiency virus who are receiving long-term combination antiretroviral therapy: results from the Adolescent Master Protocol of the Multicenter Pediatric HIV/AIDS Cohort Study. JAMA Pediatr 2013;167:520-7. [Crossref] [PubMed]
- Okoromah CA, Ojo OO, Ogunkunle OO. Cardiovascular dysfunction in HIV-infected children in a sub-Saharan African country: comparative cross-sectional observational study. J Trop Pediatr 2012;58:3-11. [Crossref] [PubMed]
- Namuyonga J, Lubega S, Musiime V, et al. Cardiac Dysfunction Among Ugandan HIV-infected Children on Antiretroviral Therapy. Pediatr Infect Dis J 2016;35:e85-8. [Crossref] [PubMed]
- Bode-Thomas F, Ige O, Oguche S, et al. Left ventricular mass and diastolic dysfunction in children infected with the human immunodeficiency virus. Niger J Cardiol 2014;11:8-12. [Crossref]
- Kim RJ, Rutstein RM. Impact of antiretroviral therapy on growth, body composition and metabolism in pediatric HIV patients. Paediatr Drugs 2010;12:187-99. [Crossref] [PubMed]
- Miller TL, Grant YT, Almeida DN, et al. Cardiometabolic Disease in Human Immunodeficiency Virus-Infected Children. J Cardiometab Syndr 2008;3:98-105. [Crossref] [PubMed]
- Sadoh WE. Cardiovascular abnormality in children with human immunodeficiency virus: Is there a need for cardiac screening? Nig J Cardiol 2014;11:1-2. [Crossref]
- Chelo D, Wawo E, Siaha V, et al. Cardiac anomalies in a group of HIV-infected children in a pediatric hospital: an echocardiographic study in Yaounde, Cameroon. Cardiovasc Diagn Ther 2015;5:444-53. [PubMed]
- Sims A, Frank L, Cross R, et al. Abnormal cardiac strain in children and young adults with HIV acquired in early life. J Am Soc Echocardiogr 2012;25:741-8. [Crossref] [PubMed]
- Fisher SD, Lipshultz SE. Epidemiology of cardiovascular involvement in HIV disease and AIDS. Ann N Y Acad Sci 2001;946:13-22. [Crossref] [PubMed]
- Ige O, Oguche S, Yilgwan C, et al. The QT interval in human immunodeficiency virus-positive Nigerian children. J Med Trop 2014;16:61-5. [Crossref]
- Miller RF, Kaski JP, Hakim J, et al. Cardiac disease in adolescents with delayed diagnosis of vertically acquired HIV infection. Clin Infect Dis 2013;56:576-82. [Crossref] [PubMed]
- Fisher SD, Easley KA, Orav EJ, et al. Mild dilated cardiomyopathy and increased left ventricular mass predict mortality: the prospective P2C2 HIV Multicenter Study. Am Heart J 2005;150:439-47. [Crossref] [PubMed]
- Ige OO, Oguche S, Bode-Thomas F. Left Ventricular Systolic Function in Nigerian Children with Human Immunodeficiency Virus Infection. Congenit Heart Dis 2012;7:417-22. [Crossref] [PubMed]
- Arodiwe I, Ikefuna A, Obidike E, et al. Left ventricular systolic function in Nigerian children infected with HIV/AIDS: a cross-sectional study. Cardiovasc J Afr 2016;27:25-9. [Crossref] [PubMed]
- National Health and Nutrition Examination Survey. Anthropometry procedures manual. Atlanta: Centers for Disease Control and Prevention, 2009.
- Olowu AO. Studies on Heart Failure in Sagamu. Niger J Paediatr 1993;20:29-34.
- Lopez L, Colan SD, Frommelt PC, et al. Recommendations for Quantification Methods During the Performance of a Pediatric Echocardiogram: A Report From the Pediatric Measurements Writing Group of the American Society of Echocardiography Pediatric and Congenital Heart Disease Council. J Am Soc Echocardiogr 2010;23:465-95. [Crossref] [PubMed]
- Devereux RB. Detection of left ventricular hypertrophy by M-mode echocardiography. Anatomic validation, standardization, and comparison to other methods. Hypertension 1987;9:II19-26. [Crossref] [PubMed]
- National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and Adolescents. The Fourth Report on the Diagnosis, Evaluation, and Treatment of High Blood Pressure in Children and Adolescents. Pediatrics 2004;114:555-76. [Crossref] [PubMed]
- Kampmann C, Wiethoff CM, Wenzel A, et al. Normal values of M mode echocardiographic measurements of more than 2000 healthy infants and children in central Europe. Heart 2000;83:667-72. [Crossref] [PubMed]
- Pettersen MD, Du W, Skeens ME, et al. Regression Equations for Calculation of Z Scores of Cardiac Structures in a Large Cohort of Healthy Infants, Children, and Adolescents: An Echocardiographic Study. J Am Soc Echocardiogr 2008;21:922-34. [Crossref] [PubMed]
- Lipshultz SE, Miller TL, Wilkinson JD, et al. Cardiac effects in perinatally HIV-infected and HIV-exposed but uninfected children and adolescents: A view from the United States of America. J Int AIDS Soc 2013;16:18597. [Crossref] [PubMed]
- Lang RM, Bierig M, Devereux RB, et al. Recommendations for chamber quantification: A report from the American Society of Echocardiography’s guidelines and standards committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiograph. J Am Soc Echocardiogr 2005;18:1440-63. [Crossref] [PubMed]
- Badal S, Gupta R, Kumar P, et al. Cardiac Manifestations in HIV Infected Children. Are they under Diagnosed. HIV/AIDS Res Treat Open J 2015;2:21-6.
- Singh P, Hemal A, Agarwal S, et al. Cardiac manifestations in HIV infected children. Indian J Pediatr 2015;82:230-4. [Crossref] [PubMed]
- Lubega S, Zirembuzi GW, Lwabi P. Heart disease among children with HIV/AIDS attending the paediatric infectious disease clinic at Mulago Hospital. Afr Health Sci 2005;5:219-26. [PubMed]
- Wilkinson JD, Williams PL, Leister E, et al. Cardiac Biomarkers in HIV-Exposed Uninfected Children: The Pediatric HIV/AIDS Cohort Study (PHACS). AIDS 2013;27:1099-108. [Crossref] [PubMed]
- Rajeshwari K, Amritsinh SP, Mandal RN, et al. Cardiac Abnormalities in HIV Infected Children Presenting to a Tertiary Level Teaching Hospital at New Delhi. Br J Med Med Res 2014;4:237-43. [Crossref]
- Pongprot Y, Sittiwangkul R, Silvilairat S, et al. Cardiac manifestations in HIV-infected Thai children. Ann Trop Paediatr 2004;24:153-9. [Crossref] [PubMed]
- Shah I, Prabhu SS. Cardiac dysfunction in HIV infected children: a pilot study. Indian Pediatr 2005;42:146-9. [PubMed]
- Idris NS, H, Cheung MM, Grobbee DE, et al. Cardiac effects of antiretroviral-naive versus antiretroviral-exposed HIV infection in children. PLoS One 2016;11:e0146753. [Crossref] [PubMed]
- Tudor AM, Anca I. Echocardiographic Aspects in HIV Infected/AIDS Children and Adolescents. Ther Pharmacol Clin Toxicol 2009;13:300-5.
- Foster BJ, MacKie AS, Mitsnefes M, et al. A novel method of expressing left ventricular mass relative to body size in children. Circulation 2008;117:2769-75. [Crossref] [PubMed]
- Lipshultz SE, Easley KA, Orav EJ, et al. Cardiac dysfunction and mortality in HIV-infected children: The Prospective P2C2 HIV Multicenter Study. Pediatric Pulmonary and Cardiac Complications of Vertically Transmitted HIV Infection (P2C2 HIV) Study Group. Circulation 2000;102:1542-8. [Crossref] [PubMed]
- Ganau A, Devereux RB, Roman MJ, et al. Patterns of left ventricular hypertrophy and geometric remodeling in essential hypertension. J Am Coll Cardiol 1992;19:1550-8. [Crossref] [PubMed]
- Lipshultz SE, Orav EJ, Sanders SP, et al. Immunoglobulins and left ventricular structure and function in pediatric HIV infection. Circulation 1995;92:2220-5. [Crossref] [PubMed]
- Lipshultz SE, Easley KA, Orav EJ, et al. Left ventricular structure and function in children infected with human immunodeficiency virus: the prospective P2C2 HIV Multicenter Study. Pediatric Pulmonary and Cardiac Complications of Vertically Transmitted HIV Infection (P2C2 HIV) Study Group. Circulation 1998;97:1246-56. [Crossref] [PubMed]
- Lipshultz SE, Wilkinson JD, Thompson B, et al. Cardiac Effects of Highly Active Antiretroviral Therapy in Perinatally HIV-Infected Children: THE CHAART-2 Study. J Am Coll Cardiol 2017;70:2240-7. [Crossref] [PubMed]
- Silva ML, Nassar SM, Silva AP, et al. Biventricular diastolic function assessed by Doppler echocardiogram in children vertically infected with human immunodeficiency virus. J Pediatr (Rio J) 2014;90:403-7. [Crossref] [PubMed]
Cite this article as: Animasahun BA, Diaku-Akinwumi IN, Ubuane PO, Ibitoye E. Cardiac size and systolic function of HIV-infected Lagos children accessing routine care: a pilot study. J Xiangya Med 2018;3:14.


