Superior vena cava (SVC) resection and reconstruction in non-small cell lung cancer (NSCLC) invasion
Introduction
Locally advanced non-small cell lung cancer (NSCLC) is a heterogeneous group and carries a dismal long-term survival. The optimal treatment (whether definitive chemoradiotherapy or neoadjuvant therapy followed by aggressive surgery) for these patients is still not uniform in view of the limited level I evidence to guide therapeutic decisions. Moreover, the role of aggressive surgery to address the local recurrence in those patients who relapse following definitive chemoradiotherapy has also been questioned considering significant perioperative morbidity and mortality, and poor survival rates. One specific circumstance of locally advanced disease is superior vena cava (SVC) involvement in NSCLC. This constitutes T4 disease and is associated with a poor prognosis. The surgical management of these patients poses a therapeutic challenge due to the anticipated risk of incomplete resection, significant perioperative complications, and concern regarding the long-term results. However, the last 15-years witnessed many reports of successful SVC resection in NSCLC with acceptable perioperative morbidity and mortality, and a good survival outcome in appropriately selected patients. Accordingly, the eighth edition of the American Joint Committee on Cancer (AJCC) classification of NSCLC classifies T4 tumors as stage III disease (1). The present review article aims to describe the rationale, the surgical techniques, and the outcomes in patients with NSCLC involving the SVC.
Methods
A PubMed search using the keyword search strategy—(((((SVC[Title/Abstract]) OR “Superior vena cava”[Title/Abstract]) OR Cava[Title/Abstract]) OR “vena cava”[Title/Abstract])) AND “Carcinoma, Non-Small-Cell Lung”[Mesh], undertaken on 20th December 2017, yielded 116 articles. Ninety-one of these articles were published in English language. A total of 24 articles were selected for the full text review following screening of abstracts of these 91 articles to identify the role and technique of SVC resection in NSCLC further. We also reviewed the articles identified by perusal of the references of these articles.
Rationale of SVC resection in NSCLC
There are two issues involved when a patient with NSCLC infiltrating the SVC is encountered. First, the technical aspect of the procedure and its attendant perioperative morbidity and mortality; and second, the oncological benefit attributable to the procedure. Table 1 displays the previously reported cases of SVC resection in NSCLC (2-16,18-20)
Table 1
| Case No. | Authors | Research question | Study design | Publication year | SVC resection | Key findings |
|---|---|---|---|---|---|---|
| 1 | Dartevelle et al. (2) | To describe the technical aspect and results of SVC resection | Retrospective analysis | 2017 | SVC resection in 50 patients (primary closure or pericardial patch plasty in 6, PTFE graft in 44) | Complete R0 resection rate: 89%; post-operative 30-day mortality: 8%. Graft patency: 88% at 6 months. Median survival: 23 months; overall, 5- and 10-year survival rates were 36.7% and 32.1%, respectively |
| 2 | Casiraghi et al. (3) | To evaluate the feasibility of salvage surgery after definitive chemoradiotherapy | Retrospective analysis | 2017 | SVC resection in 2 of 27 patients who had complete resection | With a median follow-up of 13 months, postoperative 2- and 3-year survival rates after complete resection were 46% and 37%, respectively. Salvage lung resection after definitive chemoradiation therapy is feasible, with acceptable postoperative survival and complication rates |
| 3 | Zhu et al. (4) | To report an unusual case of non-small cell lung cancer (NSCLC) invading the SVC accompanying PLSVC | Case report | 2015 | One | First report of a successful complete resection of locally advanced lung cancer involving SVC, right pulmonary artery trunk and main bronchus with persistent left SVC |
| 4 | Kusumot et al. (5) | To assess the surgical indications for non-small cell lung cancer (NSCLC) infiltrating a great vessel or the heart | Retrospective analysis | 2015 | SVC resection in 5 of 14 patients: ringed graft in 3, direct closure in 1 | Five-year survival from SVC resection: 20% with median survival of 26 months |
| 5 | Windisch et al. (6) | To analyse the clinical course and survival following surgical intervention in lung cancer infiltrating SVC | Retrospective analysis | 2015 | SVC resection in 22 patients: ringed graft in 17, direct closure in 5 | Five-year survival probability was 33 % for patients with SVC reconstruction and 25 % for patients with SVC replacement (P= 0.22) |
| 6 | Sun et al. (7) | To describe a simultaneous triple plasty on the SVC, pulmonary artery (PA), and main bronchus for central-type lung cancers | Retrospective analysis | 2013 | SVC resection in four patients: ringed graft in 1, direct closure in 3 | Triple plasty of bronchus, PA, and SVC is both practical and safe for patients with locally advanced NSCLC. For patients with strict indications, the long-term survival is favorable. |
| 7 | Durkovic et al. (8) | To describe azygo-atrial bypass following SVC resection in a patient with NSCLC and SVC syndrome | Case report | 2012 | SVC resection, Prosthetic azygo-atrial bypass | A technique of SVC reconstruction |
| 8 | Shinohara et al. (9) | To describe a technique of resection-reconstruction of SVC in NSCLC | Case report | 2009 | SVC resection followed by ringed graft in one patient | A technique of SVC reconstruction |
| 9 | Lanuti et al. (10) | To analyze the operative results, graft patency, and survival in patients undergoing SVC resection and reconstruction | Retrospective analysis | 2009 | SVC resection in 17 patients: ringed graft in 12, direct closure in 5 (pericardial patch in 3) | Five-year survival probability of patients with resected lung cancer: 30% |
| 10 | Yildizeli et al. (11) | To assess operative mortality, morbidity, and long-term results of patients with surgically resected T4 non-small cell lung carcinoma | Retrospective analysis | 2008 | SVC resection in 39 patients | Overall 5-year survival rate for SVC resection: 29.4% |
| 11 | Borri et al. (12) | To demonstrate the postoperative risk and oncological benefits of extended pneumonectomy | Retrospective analysis | 2007 | SVC resection in 13 of 47 patients (combined with carinal resection in 9) | Overall 60-day mortality was 8.5%. Major postoperative complications occurred in 8 patients (17%). The 2- and 5-year survival rates for the overall population were 42% and 22.8%, respectively |
| 12 | Lucchi et al. (13) | To study the indications of extended resection in locally advanced NSCLC | Retrospective analysis | 2007 | SVC resection in four patients | Surgery for T4 NSCLC may be effective in those patients without mediastinal (N2) lymph node involvement |
| 13 | Politi et al. (14) | Comparison of two techniques of SVC reconstruction following resection in patients with locally advanced NSCLC and thymoma | Retrospective analysis | 2007 | SVC resection in 32 patients with NSCLC: PTFE graft in 16, direct closure in 16 | Mean survival of 23 months in those patients (combined T4 NSCLC and thymoma) undergoing PTFE replacement. Mean survival of 15 months in patients undergoing tangential resections for NSCLC with extracapsular N2 |
| 14 | Misthos et al. (15) | The authors studied the role of surgical treatment in case of direct aortic and superior venous caval involvement. | Retrospective analysis | 2006 | SVC resection in 9 patients: primary closure in 3, Dacron patch in 5, and vascular graft in 1) | There was no postoperative mortality. The survival ranged from 10 to 60 months (Fig. 4) and median 5-year survival was 31±17 months. The 5-year survival was 11% |
| 15 | Spaggiari et al. (16) | To describe SVC reconstruction with a heterologous bovine custom-made pericardium tube | Case report | 2004 | One | A case of SVC revascularization successfully performed with heterologous “custom-made” pericardial tube. This type of revascularization may improve the reconstruction of large mediastinal veins after their resection for malignancies |
| 16 | Suzuki et al. (17) | To clarify the surgical outcome of combined resection and reconstruction of the SVC for NSCLC | Retrospective analysis | 2004 | SVC resection in 40 patients: partial in 29 (direct running closure in 21, autologous pericardial patch in 8), complete in 11 followed by vascular graft | Significant survival difference between patients with SVC invasion by metastatic lymph nodes and those with SVC invasion by a direct tumor extension (5-year survival rate: 6.6% versus 36%) |
| 17 | Shargall et al. (18) | To examine our results with surgery for locally advanced non-small cell lung cancer (NSCLC) invading the superior vena cava (SVC) | Retrospective analysis | 2004 | SVC resection in 15 patients: primary closure in 4, pericardial patch in 2, and prosthetic graft in 9) | There were two postoperative deaths (14%) and three major morbidities (23%). There was one late graft thrombosis. Mean follow-up was 25 months (range, 3–132, median 11 months). Overall 1- and 3-year survival was 68% and 57% and disease-free survival was 55 and 27%, respectively |
| 18 | Spaggiari et al. (19) | To identify prognostic factors for SVC resection in NSCLC | Multicentre, retrospective analysis | 2004 | SVC resection in 109 patients: simple running suture closure in 58, vascular stapler in 14, pericardial patch in 5, PTFE patch in 3, and prosthetic replacement in 29 | Major postoperative morbidity and mortality were 30% and 12%, respectively. Five-year survival was at 21%, with median survival at 11 months. In multiple regression analysis, induction treatment was associated with an increased risk of major complications |
| 19 | Doddoli et al. (20) | To assess the results of the surgical treatment of patients with stage IIIB non-small cell lung carcinoma (NSCLC) invading the mediastinum (T4) | Retrospective analysis | 2001 | SVC resection in 17 patients: partial in 13 (direct running closure in 11, PTFE patch in 2), complete in 4 followed by PTFE graft | Surgical management of T4 NSCLC invading the mediastinum should be considered, in the absence of N2 disease, when a complete resection is achievable |
PLSVC, persistent left superior vena cava; SVC, superior vena cava; PA, pulmonary artery.
The technical success and acceptable perioperative morbidity and mortality of the SVC resection in locally advanced NSCLC were reported in a large case series of 109 patients (17). This multicenter retrospective study reported that 30% of the patients had major postoperative complications and 12% of them died. The cause of postoperative death included pulmonary edema, post-pneumonectomy pulmonary edema, bronchial fistula, lung abscess and embolism. Another study of 39 SVC resections in a case series of 271 patients with T4 tumors also highlighted the technical feasibility of SVC resection with acceptable morbidity (n=4, 10.3%) and mortality (n=3, 7.7%) (10).
The long-term results of the SVC resection in NSCLC are likely to be influenced by a number of factors – the extent of surgery, reconstruction of SVC, and the pattern of invasion (due to either direct invasion by the tumor or secondary to enlarged mediastinal lymph nodes). Various studies reported that almost one fourth of the NSCLC patients undergoing SVC resection survived for 5 years (4,5,9-11).
A multivariate analysis of 109 patients with NSCLC undergoing SVC resection showed that two factors influenced the survival—pneumonectomy [hazard ratio (HR), 2.9; 95% CI, 1.1–5.8; adjusted estimate P=0.0013] and complete SVC resection (HR, 2.2; 95% CI, 1.2–4.0; adjusted estimate P=0.014) (17). The authors recommended that pathological bulky N2 disease should be considered a contraindication for surgery. In otherwise selected patients of SVC resection, the use of neoadjuvant chemotherapy (NACT) may help to reduce the mediastinal invasion resulting in conservative SVC and pulmonary resections. Moreover, NACT would also allow the testing of the biological behavior of the tumor in a given patient.
The pattern of SVC invasion also significantly affects the 5-years survival in patients undergoing radical surgery. A study of 40 cases of SVC resection highlighted that there was a significant survival difference between patients with SVC invasion by metastatic lymph nodes and those with SVC invasion by direct tumor extension (5-year survival rate: 6.6% versus 36%) (20). Another study of 17 patients compared the survival outcome in patients with NSCLC undergoing SVC resection also highlighted the node-negative resections had significantly better outcomes (P=0.040) compared to node-positive resections (9). Based on these studies, it may be concluded that SVC resection may not be a worthwhile surgical exercise in the setting of bulky N2 disease infiltrating the SVC.
Patient selection criteria
The ideal patients for this procedure are those who have direct tumor infiltration of the SVC and have N0/N1 disease (stage IIIA, AJCC 8th edition). Furthermore, a subgroup of patients having partial resection of SVC and right upper lobectomy are likely to have good perioperative outcome and long-term survival. Those patients who have N2 disease (stage IIIB, AJCC 8th edition) and/or are anticipated to have pneumonectomy are better treated with NACT first, followed by surgery if pathologic downstaging has occurred.
Preoperative workup
As SVC resection carries a significant perioperative morbidity and mortality, it is needless to mention that a diligent preoperative planning is essential to have a fruitful patient outcome. A whole-body positron-emission tomography-computed tomography (PET-CT) must be done in all these patients to rule out metastatic disease before embarking upon SVC resection. Brain MRI should also be undertaken to rule out brain metastases; it also helps to exclude any other brain disease that may be worsened while SVC is clamped during the surgery. A contrast enhanced CT scan is helpful in assessment of the SVC and innominate veins, the presence of intra-luminal thrombus and the associated venous collateralization. A magnetic resonance imaging may also be used for better depiction of vascular and pericardial involvement. A meticulous mediastinal staging should be done using endobronchial ultrasound or careful mediastinoscopy to rule out extensive N2 disease which precludes the resection of SV (21).
Operative procedure
The patient is intubated with double lumen endotracheal tube. One should not place any indwelling venous catheters in the subclavian or the jugular veins in a planned SVC resection or remove these catheters if already present. A femoral central venous line serves to provide access to the inferior vena cava system in the likely scenario that the superior system has to be clamped for resection and reconstruction.
Surgical approach
The surgical approach to the patients with NSCLC with SVC infiltration includes right posterolateral thoracotomy, median sternotomy, or right hemi-clamshell approach. A routine right posterolateral thoracotomy for right lung resection done through the 4th or 5th intercostal space provides sufficient exposure of the SVC for resection. The 4th intercostal space may be preferred if a more proximal control over the right or the left innominate vein is anticipated. A median sternotomy may be opted in cases where a cardiopulmonary bypass, though rarely, is required or the left innominate vein needs to be reconstructed (19,21). The limitations of median sternotomy are difficult subcarinal node dissection and intraparenchymal fissure management. A right hemiclamshell approach provides an excellent exposure of the SVC, both the right and the left innominate veins, and the right subclavian veins. Moreover, it provides a good access to right posterior hemithorax. This approach may be of help when dealing with (I) a large tumor requiring a chest wall resection or having dense adhesions posteriorly, (II) tumor requiring right upper lobe sleeve resection or a right pneumonectomy.
Reconstruction options
The reconstruction of the SVC depends upon the circumference resected. If the resected SVC is less than 50% of the circumference, a primary or patch closure can be undertaken for the SVC reconstruction. However, it is advisable to achieve a primary closure in a small SVC defect to avoid its narrowing and kinking. Either bovine or autologous pericardial patch can be used whenever the primary closure is deemed difficult. If the resected SVC is likely to be more than 50% of the circumference, it is advisable to resect the segment of the cava followed by reconstruction using a polytetrafluoroethylene (PTFE) ringed graft (median size 14; range, 8–18). Other options for SVC reconstruction following complete resection are tubularized autologous/bovine pericardium, spiral saphenous vein graft, and cryo-preserved arterial allografts (9).
When there is involvement of the proximal SVC, the prosthetic graft may be anastomosed proximally to the right innominate vein after suture ligating the left innominate vein. When tumor involvement extends into the right innominate vein, a graft may be placed between the left innominate vein and the right atrium, followed by SVC resection and suture ligation of the right innominate vein. Preservation of one of the innominate venous drainages is sufficient as the unilateral arm swelling observed with the transection of one innominate vein resolves over time.
SVC reconstruction is not required in cases of extensive thrombosis of the subclavian veins or in the presence of well-developed venous collaterals. Simple suture ligation of the SVC ends is sufficient following its resection in these scenarios. Durkovic et al. described a prosthetic azygo-atrial bypass in a patient who underwent segmental resection of SVC for palliation of SVC syndrome (8).
Surgical technique
During exploration, if it is found that the SVC is involved, initial efforts should be made to obtain proximal control of the SVC and the innominate veins. Distal control of the SVC warrants the division of the superior pulmonary vein first and then, the control of the superior pulmonary artery (PA) and the truncus arteriosus. A rare involvement of the PA may mandate its intra-pericardial control between the SVC and the ascending aorta. Following these maneuvers, vascular control of the distal SVC below the azygous vein can be achieved.
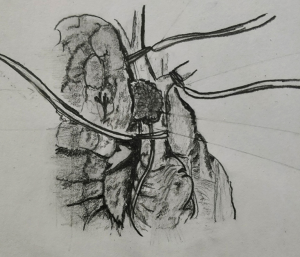
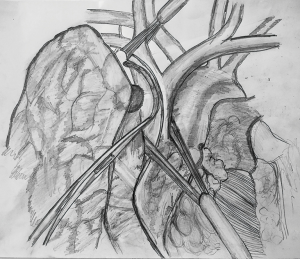
If the anticipated resection of the SVC is less than 50% of its circumference, SVC should be mobilized as much as possible before placing a tangential vascular clamp (Figure 1). A primary or patch closure is achieved using a 4-0 polypropylene suture. If a segment of SVC is resected in view of more than 50% circumferential involvement by the tumor, intravenous heparin is administered before the clamps are applied to the proximal and the distal part (Figure 2). SVC cross clamping initiates several adverse hemodynamic effects: (I) decreased right ventricular preload resulting in decreased cardiac output and eventual systemic hypotension, and (II) increased jugular venous pressure that can increase the risk of intracranial thrombosis and edema (22). A number of maneuvers may be used to manage the temporary interruption of flow during the time of SVC clamping—intravascular fluid expansion and vasopressors to maintain the blood pressure, lower extremity venous access, hyperventilation to reduce cerebral edema, and reverse Trendelenburg position (9). SVC cross clamping of 30–60 minutes is well tolerated by most of the patients. Four-zero polypropylene suture is used for the anastomosis of the graft to the recipient.
Postoperative care
A number of specific complications may be associated with the resection and reconstruction of the SVC. Anastomotic stenosis and graft thrombosis are potential concerns in the postoperative patient related to SVC reconstruction. Anastomotic stenosis may occur both at the level of the proximal and distal suture lines due to improper positioning of the graft or technical error (23). An early diagnosis may call for a revisional surgery; however, dilation and stenting may help if it is diagnosed later. A variable incidence of graft thrombosis following complete SVC resection is reported in various studies ranging from 0–24% (10,17). The reasons for graft thrombosis may be related to reduced blood flow through the prosthesis due to the presence of collaterals, or the use of small lumen prosthesis (17). Strict anticoagulation for up to 6 months and maintaining high flow through the grafts must be instituted in all the patients to prevent graft thrombosis (19). A partial SVC resection does not require postoperative anticoagulation.
Conclusions
The resection of SVC in a locally advanced NSCLC is feasible with an acceptable perioperative morbidity and mortality in a carefully selected patient. There is a need for prospective studies to assess the oncological benefit of these radical resections.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: The article was commissioned by the editorial office, Journal of Xiangya Medicine for the series “Extended Pulmonary Resections for T4 Non-Small-Cell-Lung-Cancer”. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jxym.2018.04.02). The series “Extended Pulmonary Resections for T4 Non-Small-Cell-Lung-Cancer” was commissioned by the editorial office without any funding or sponsorship. S. Yendamuri served as the unpaid Guest Editor of the series. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Detterbeck FC, Boffa DJ, Kim AW, et al. The Eighth Edition Lung Cancer Stage Classification. Chest 2017;151:193-203.
- Dartevelle PG, Mitilian D, Fadel E. Extended surgery for T4 lung cancer: a 30 years’ experience. Gen Thorac Cardiovasc Surg 2017;65:321-8. [Crossref] [PubMed]
- Casiraghi M, Maisonneuve P, Piperno G, et al. Salvage Surgery After Definitive Chemoradiotherapy for Non-small Cell Lung Cancer. Semin Thorac Cardiovasc Surg 2017;29:233-41. [Crossref] [PubMed]
- Zhu D, Qiu X, Zhou Q. Combined Double Sleeve Lobectomy and Superior Vena Cava Resection for Non-small Cell Lung Cancer with Persistent Left Superior Vena Cava. Zhongguo Fei Ai Za Zhi 2015;18:718-20. [PubMed]
- Kusumoto H, Shintani Y, Funaki S, et al. Combined resection of great vessels or the heart for non-small lung cancer. Ann Thorac Cardiovasc Surg 2015;21:332-7. [Crossref] [PubMed]
- Windisch T, Fischer JR, Vega A, et al. Infiltration of the superior vena cava in NSCLC: results of surgical intervention. Pneumologie 2015;69:23-9. [PubMed]
- Sun Y, Zheng H, Chen Q, et al. Triple plasty of bronchus, pulmonary artery, and superior vena cava for non-small cell lung cancer. Ann Thorac Surg 2013;95:420-4. [Crossref] [PubMed]
- Durkovic S, Di Chiara F, Rea F. Prosthetic azygo-atrial bypass for palliation of superior vena cava syndrome. Eur J Cardiothorac Surg 2012;41:e56-8. [Crossref] [PubMed]
- Shinohara H, Tsuchida M, Hashimoto T, et al. Superior vena cava reconstruction via a posterolateral thoracotomy without venous occlusion for locally advanced lung cancer: report of a case. Surg Today 2009;39:787-9. [Crossref] [PubMed]
- Lanuti M, De Delva PE, Gaissert HA, et al. Review of superior vena cava resection in the management of benign disease and pulmonary or mediastinal malignancies. Ann Thorac Surg 2009;88:392-7. [Crossref] [PubMed]
- Yildizeli B, Dartevelle PG, Fadel E, et al. Results of primary surgery with T4 non-small cell lung cancer during a 25-year period in a single center: the benefit is worth the risk. Ann Thorac Surg 2008;86:1065-75. [Crossref] [PubMed]
- Borri A, Leo F, Veronesi G, et al. Extended pneumonectomy for non-small cell lung cancer: morbidity, mortality, and long-term results. J Thorac Cardiovasc Surg 2007;134:1266-72. [Crossref] [PubMed]
- Lucchi M, Viti A, Melfi F, et al. IIIB-T4 non-small cell lung cancer: indications and results of surgical treatment. J Cardiovasc Surg (Torino) 2007;48:369-74. [PubMed]
- Politi L, Crisci C, Montinaro F, et al. Prosthetic replacement and tangential resection of the superior vena cava in chest tumors. J Cardiovasc Surg (Torino) 2007;48:363-8. [PubMed]
- Misthos P, Papagiannakis G, Kokotsakis J, et al. Surgical management of lung cancer invading the aorta or the superior vena cava. Lung Cancer 2007;56:223-7. [Crossref] [PubMed]
- Spaggiari L, Veronesi G, D'Aiuto M, et al. Superior vena cava reconstruction using heterologous pericardial tube after extended resection for lung cancer. Eur J Cardiothorac Surg 2004;26:649-51. [Crossref] [PubMed]
- Suzuki K, Asamura H, Watanabe S, et al. Combined resection of superior vena cava for lung carcinoma: prognostic significance of patterns of superior vena cava invasion. Ann Thorac Surg 2004;78:1184-9. [Crossref] [PubMed]
- Shargall Y, de Perrot M, Keshavjee S, et al. 15 years single center experience with surgical resection of the superior vena cava for non-small cell lung cancer. Lung Cancer 2004;45:357-63. [Crossref] [PubMed]
- Spaggiari L, Magdeleinat P, Kondo H, et al. Results of superior vena cava resection for lung cancer. Analysis of prognostic factors. Lung Cancer 2004;44:339-46. [Crossref] [PubMed]
- Doddoli C, Rollet G, Thomas P, et al. Is lung cancer surgery justified in patients with direct mediastinal invasion? Eur J Cardiothorac Surg 2001;20:339-43. [Crossref] [PubMed]
- Jones DR. Technique of Superior Vena Cava Resection for Lung Carcinomas. Oper Tech Thorac Cardiovasc Surg 2008;13:274-82. [Crossref]
- Lee D-SD, Flores RM. Superior Vena Caval Resection in Lung Cancer. Thorac Surg Clin 2014;24:441-7. [Crossref] [PubMed]
- Grunenwald DH. Resection of lung carcinomas invading the mediastinum, including the superior vena cava. Thorac Surg Clin 2004;14:255-63. [Crossref] [PubMed]
Cite this article as: Garg PK, Sharma S, Arya S, Yendamuri S. Superior vena cava (SVC) resection and reconstruction in non-small cell lung cancer (NSCLC) invasion. J Xiangya Med 2018;3:13.